Methods of absolute (direct and chronometric) age determination : Part 1

Introduction
Absolute dating methods are also referred to as chronometric dating methods. Absolute chronology or the direct determination of time tries to ascertain absolute age, an age which can be measured in definite units of time. The absolute age of objects and/or industrial complexes are expressed in units of time of the Christian calendar, which is an absolute scale of time that is based on units of time which have been determined astronomically. Wherever there are (written) documents containing dates which can be linked with this calendar, we speak of absolute periods of time or absolute chronology.
Absolute dating

Absolute dating provides specific dates or ranges of dates, in years. Examples of absolute dating techniques include dendrochronology, radiocarbon, and potassium-argon dating.
Strictly speaking, the term "absolute" only applies when the age of an object can be determined per se, as for example, when the date on which it was struck appears on a coin, or when the age of organic material can be accurately established by the 14C method. The term "absolute" is, however, also applied in a broader sense to objects of which the absolute chronological age is accepted as being fairly certain.
The Radiocarbon or 14C method

The 14C method enables us to make direct determination of the absolute age of organic material such as charcoal, bones, wood, grass, peat and shells by studying the radioactivity of carbon.

WF Libby
This method was developed in 1949 by an American scientist, WF Libby. It is reliable for objects not older than 30 000 - 40 000 years. The time range of radiocarbon dating can, however, be extended to about 70 000 years by means of isotopic enrichment. This involves the adding of modern carbon to the archaeological carbon sample to be dated. With the AMS (accelerator mass spectrometry) technique it is now possible to date minute samples - to the extent that a single grain of wheat or a seed can be dated (Renfrew & Bahn 1996).
When Libby originally formulated the radiocarbon dating method, he incorrectly assumed that the concentration of 14C in the atmosphere had never fluctuated in the past. Radiocarbon dates therefore have to be calibrated to account for the fluctuations which did occur, partly as a result of changes in the strength of the earth's magnetic field, the burning of oil and coal,and the testing of nuclear weapons. Fortunately, accurate tree-rings (dendrochronology) can be used to correct or calibrate radiocarbon dates covering the past 8000 years.
The Scientific Principles on which the method is based.
~Every living organism contains radioactive carbon (14C) which it absorbs directly or indirectly throughout its life-span. (14C is a radioactive isotope of carbon contains eight instead of the usual six neutrons in the nucleus.)
~This radiocarbon is absorbed in the form of radioactive carbon dioxide which is found in the biosphere in addition to ordinary carbon (14C).
~It is formed by cosmic radiation which produces neutrons: these neutrons are then changed into radioactive carbon as a result of their reaction with nitrogen.
~14C combines with oxygen in the atmosphere to form carbon dioxide and radioactive carbon is absorbed in this form by plants through the process of photosynthesis.
~Animals live from plant material, therefore radiocarbon is present in all living organic material.
~The ratio between 14C and 12C in all living organic material is the material is the same as that in the atmosphere.
~The is a constant disintegration in the radiocarbon but new 14C atoms, immediately absorbed by the living organism, are produced equally fast. The ratio between 14C and 12C in a living organism thus remains constant.
~When a living organism dies, the absorption of 14C atoms ceases and a result of the abovementioned disintegration the 14C gradually decreases.
~After 5730 +-40 years (called the half life of 14C), only half of the 14C is left, another 5730 +-40 years only one-forth of the original, etctetera until all radiocarbon has disappeared or can no longer be measured. (The half-life of 14C was originally calculated at 5568 +-30years, but it was discovered later that 5730 +-40years represent a more accurate value.)
~The disintegration takes place at a fixed rate. By counting, with a Geiger counter for example, the number disintegrations that take place per unit of time in a given quantity of organic material, one can calculate how long ago the organism lived.
~In the accelerator mass spectrometric (AMS) technique the proportion of 14C to 12C atoms is directly measured. Very small quantities of datable material are used and it is therefore not such a destructive technique. This allows for the dating of sensitive archaeological material or historical objects like the Turin shroud.
~Such a date can be expressed as follows: 1500 +-150 Before Present (BP). The standard deviation of +-150 applied to the above mentioned date indicates that there is a 67% possibility that the correct date for the particular object (organism) falls between 1350 and 1650 Before Present.
~The year AD 1950 is taken as the present in all 14C dates.
~The value of the standard deviation (or statistical error) applied to 14C dates varies with different dates. The standard deviation (sigma) of +-180 years in the date of 3621 +- 180 years before the (BP) referred to by (Fagan) is therefore merely an example.It reflects the variation in the different counts of the radiocarbon sample and indicates that there is a 67% possibility that the correct date for a particular object falls between 3441 and 3801 years before the present.
~Also remember that AD 1950 is taken as the present in all 14C dates.

The collection of datable archaeological samples, the laboratory procedures and the limitations of the method.
~Note that 14C datings are usually done by physicists (ie:@lemouth,@locikll who offices are air conditioned in Paris) and that archaeologists's (ie:@zest, my office is a deep dirt hole, may be if I am lucky a cool cave in Africa) main task is to ensure that the datable material, for example wood charcoal, is collected in a reliable manner from undisturbed deposits.
~The stratigraphic context of the datable material should therefore be established beyond all doubt.
~Care should also be taken not contaminate the datable material by introducing younger carbon (from paper bags for example)into it by packaging.
~As indicated above, the 14C method is reliable for objects not older than 30 000 - 40 000 years.

Applications in Archaeology
Radiocarbon is most commonly used dating method. It can be used in any part of the world on any material of organic or living origin. This method can be used to date organic material in the time range 40 000 years to potentially 80 000 years ago, with the aid of the AMS technique. Radiocarbon dating by means of AMS can be used to date precious objects as well as very small samples. The AMS method has been used, for example to re-evaluate all early radiocarbon dates for the introduction of sheep into southern Africa. Previous estimates were obtained from associated objects such as charcoal from sites where the sheep bones were significantly different and in most cases appreciably younger.
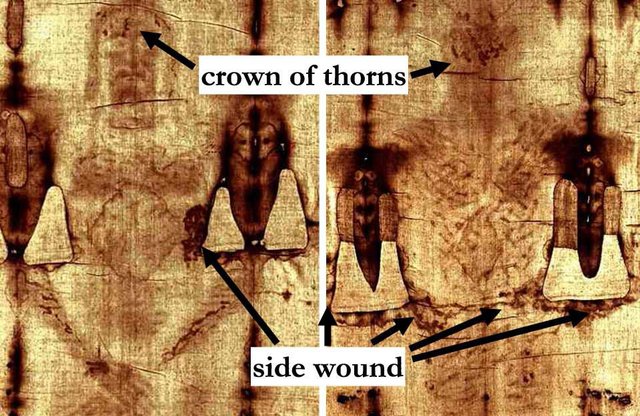
Turin Mystery : Small samples from the Shroud of Turin, said to be the burial cloth of Jesus Christ, were dated by accelerator mass spectrometry providing conclusive evidence that the linen of the shroud is medieval.
No Smoking at QUADRU
Some archaeologist prohibit smoking near an excavation, as this may result in contamination of 14C samples. I am that Archaeologist.
Thank you reading
References: Renfrew & Bahn 1996
Fagan
Part 2 to follow.....
Please Follow me for more on Archaeology and History.
Please check out my other posts:
Methods of Relative (Indirect) Age Determination used in Archaeology : Part 3
Methods of Relative (Indirect) Age Determination used in Archaeology : Part 2
Methods of Relative (Indirect) Age Determination used in Archaeology : Part 1 - Introduction
The Preservation of an Archaeological Site : Part 2
The Preservation of an Archaeological Site : Part 1
Discovering your own Archaeological Site : Part 3
Discovering your own Archaeological Site : Part 2
Discovering your own Archaeological Site : Part 1
Archaeological Sealed Sites and River Deposits : Is it an Archaeological Site or Not? - Part 3
Caves, Rock Shelters and Larger Open-Air Sites : Is it an Archaeological Site or Not? - Part 2
Is it an Archaeological Site or Not? - Part 1
Understanding the Archaeological Record : The Aims and Subject Matter of Archaeology - Part 2
The Aims and Subject Matter of Archaeology - Part 1
Archaeology and the Natural Sciences
Introduction to Ethnographic Analogy and Ethnoarchaeology
The Nature and Scope of Archaeology
The Three - Age System : The Stone Age, The Bronze Age and The Iron Age
The Roots of Modern Archaeology
Significant 18th and 19th Centuries Discoveries in Archaeology
Archaeology as a Profession- Part 2
Archaeology as a Profession- Part 1
To Become or Not Become an Archaeologist? - Introduction to Archaeology Part 2
Please Upvote and Resteem.
Thank You!
Upvoted and Resteemed by xx-votesplus, the dropAhead curation team!
Do you want more earnings?
By doing things above you will give us more STEEM POWER (SP) to give you more earnings.
Keep up the good work!
Most recent post: Moving #25_votes_plus to Discord
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hi @mandolincarls, Thank you curating and promoting my post!!!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This post has received a 2.56 % upvote from @buildawhale thanks to: @trumpman. Send 0.100 or more SBD to @buildawhale with a post link in the memo field to bid on the next vote.
To support our curation initiative, please vote on my owner, @themarkymark, as a Steem Witness
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @zest! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPDownvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I am really enjoying reading these posts!
A small issue:
Strictly speaking, the 14C in the atmosphere is decaying at the same rate as 14C in organisms, and this is why living organisms have the same ratio of 12C/14C as the current atmosphere. If the ratio in the atmosphere changes (and it is changing), then living organisms maintain that same ratio, not a constant ratio.
Thanks!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit