The Atom
The elements of the nature and the bioelements
The bioelements are the chemical components, that the alive beings are a part, these elements are abundant in the alive matter as: hydrogen (H), the oxygen (O), the carbon (C), and the nitrogen (N), to new paragraph it is possible to find also, sulfur (S), phosphorus (P), calcium (Ca), they are in minor concentration, but also they are important. Now we take the microelements an as as fluorine (F), I shoe (Faith), zinc (Zn), and the copper (Cu), only they are in the alive beings in very small concentrations, and his absence can provoke important shortcomings.
The carbon can form four linkage covalentes with it itself and with other elements, the atoms of carbon close covalentemente lead to long chain, simply branched out of rings, which contribute the skeleton on which other bioelements are distributed.
The hydrogen has an important role in the reactions of transference of energy and form together with the oxygen the water, the nitrogen also they join with the hydrogen to form functional groups in the proteins. Certain simple combinations, between the atoms of the majority cuatros of the alive beings, lead to functional or radical groups with physical and chemical properties, which influence the behavior of any molecule in which they present themselves, radical these are: methyl, hidroxilo, carboxilo, and amino.
Some bioelements are in the alive beings in form iónica, as the case of the sodium (Na), potassium (K), calcium (Ca), chlorine (Cl-), magnesium (Mg); they have important functions in the regulation of the osmotic processes or in the liberation of vesicles on the part of the cells.
The human beings, in most of the biological aspects, we are identical to the alive remains of organism, we are constituted by very similar cells, that has more or less the same chemical composition and that collaborate to form the different organs; they reproduce of way similar and take system of genetic information of the same class.
Basic Anatomofisiologia and Pathology, Written by Francisco Javier Fonseca of the Well - page 28; 2009.
so simple as the water
In a water molecule, every atom of oxygen they join with two atom of hydrogen and share an electron with each of them, this linkage covalentes forms an angle approximate mind 105 grades, the electrons remain arranged such form, that the water molecule, knowing of being globally neutral, presents asymmetry or electrical polarity, from what it is said, that the water has a polar molecule. A partial negative load is located in the atom of oxygen, whereas on the atoms of hydrogen there establish footpaths partial positive charges, the spatial distribution of electrical charges, allows the formation of bridges of hydrogen between several water molecules.
The bridges or linkage of hydrogen, they are electrostatic attractions between the partial negative charges placed on other elements, as the oxygen, as this one is forming it causes several linkage of hydrogen, the probability of which they form more they increase and the grade of cohesion of the close molecules is major, every water molecule is capable of forming bridge of hydrogen, with other cuatros water molecules, this property is the person in charge of the high internal cohesion of the liquid water.

Diagram of the molecule of the water (H2O), source of image of mastery of Wikimedia Commons
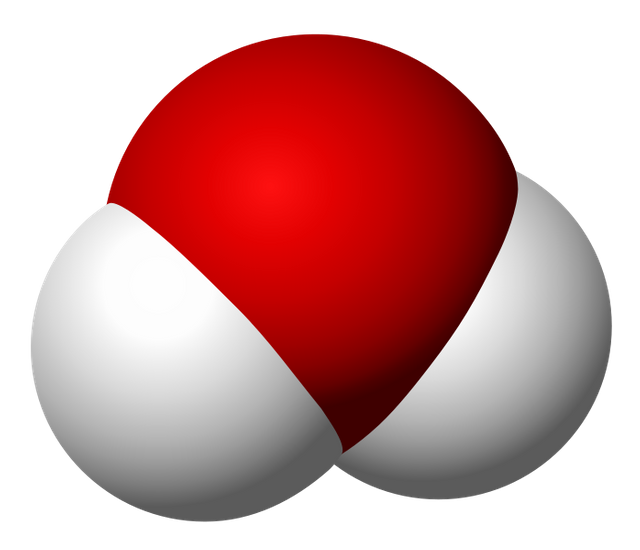
Water molecule, source of image of mastery of Wikimedia Commons
The inorganic molecules / The water
The water they represent from 70 % to 90 % of the weight of most of the alive organisms, it is a substance with properties frequent little, that the difference, so much physical, like chemistry of rest of liquids, the alive organisms have adapted themselves of forming, that can make use of the unusual properties of the water. His high specific heat they allow that some animals could regulate his corporal temperature, the high grade of internal cohesion of the water constitutes the effective way, so that the top plants could trasportar you them go out minerals in dissolution.
you Them go out minerals in dissolution they are a part of the cells and of the intercellular liquids, generally you them go out minerals they establish iónicos of supreme importance, so that the functions of permeability are carried out and contractibilidad cellular. You them go out insoluble, as the carbons and phosphate of calcium, they have a skeletal function, since they are a part of the bones of the vertebrates and of the scallops of molluscs, that's why it is said that the inorganic molecules are the water and you them go out minerals.
Secondhand Bibliography
Knife-edges. biolog: Origins of the alive Matter - Page 1; for José Ingenieros - 1910.
Basic Anatomofisiología and Pathology - Page 28; for Francisco Javier Fonseca of the Well - 2009.
Biochemistry of the metabolic processes - Page 31 for Óscar Cuamatzi Tapia - 2004.

Congratulations @dark69! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board of Honor
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @dark69! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board of Honor
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit