Hello, great readers. It’s been a while I last wrote on this great platform and It’s so wonderful to be back. Today, I’ll continue my write up on UNDERSTANDING THE AROMATIC COMPOUNDS AND ARENES. So, I will be starting with arenes in exhaust fumes.
ARENES IN EXHAUST FUMES
Petrol contains aromatic hydrocarbons, which help to improve its octane rating. In an internal combustion engine, complete combustion of the fuel (petrol or diesel) rarely occurs. Some carbon monoxide, soot and smoke is always produced. Analysis of the soot by gas-liquid chromatography and spectroscopy shows that it contains the arenes benzene, naphthalene, phenanthrene, pyrene, coronene and ovalene. The presence of benzene is of particular concern, since it is a known carcinogen.

Exhaust fumes. Image from Pixabay.
DEMAND FOR AROMATIC COMPOUNDS
The synthesis of aromatic compounds often starts with one of the arenes. Aromatic compounds are needed for new technologies and new synthetic methods, and so the demand for arenes continues to expand. Until the 1940s, the main source of commercially produced benzene was coal, but today the principal source is crude oil.
AROMATIC COMPOUNDS FROM CRUDE OIL
Benzene and other aromatic compounds form only a small proportion of crude oil, and fractional distillation does not produce sufficient benzene or other aromatic compounds to match demand. So, as in the case of alkenes, cracking and reforming are processes used to increase the supply of arenes.
Catalytic reforming
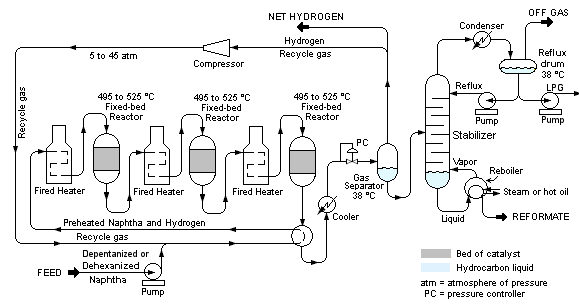
In catalytic reforming, C6 to C10 hydrocarbon vapours are heated in the presence of hydrogen at high pressure and high temperature, typically 900°C, using a transition-element catalyst.
During the reforming process, the number of carbon atoms per molecule remains the same, but a change occurs in the carbon skeleton and/or the number of hydrogen atoms per molecule. For example, cyclohexane can be reformed to give benzene, and heptane can be reformed to give methylbenzene. In the second example, note that not only are hydrogen atoms lost, but also the carbon skeleton is rearranged.
Manufacture of benzene
Naphtha is a light fraction of crude oil that contains liquid alkanes of low relative molecular mass. To make benzene, naphtha vapour is passed over a catalyst, such as platinum or molybdenum(VI) oxide, on aluminium oxide at 500 °C and 10-20 atmospheres of pressure. Compounds such as hexane found in naphtha are converted into ring compounds such as cyclohexane, which is then dehydrogenated to make benzene.
CH3CH2CH2CH2CH2CH3 → C6H12 → C6H6
ELECTROPHILIC SUBSTITUTION REACTIONS OF BENZENE
In almost all synthetic schemes that start with an aromatic hydrocarbon (such as benzene), the substitution of one or more of the hydrogen atoms occurs. This substitution is normally electrophilic, and so only those groups that can behave as electrophiles are able to replace hydrogen atoms attached to the benzene ring.
MECHANISM OF ELECTROPHILIC SUBSTITUTION
The presence of the delocalised electrons means that benzene is electron rich. Therefore, an electrophile that is able to accept an electron pair donated by the ring is the most likely type of reagent to react with benzene. This is similar to the first step in the electrophilic addition of an alkene. The resulting positive species is much more stable than a normal carbocation, since it is possible to delocalize the positive charge around the ring. A proton is then eliminated from the positive ion to restore the stability of the benzene ring. We saw the unique stability of the benzene ring when looking at the bonding in benzene. This stability is also apparent to the mechanism of electrophilic substitution.
Electrophilic substitution clearly needs an electrophile, but what is not obvious is that the electrophile must be an exceptionally good electrophile. This is because it has to react with a molecule made extremely stable by the presence of the π system of delocalized electrons. Benzene has a delocalised π system of electrons over all six atoms of the ring. But in the positive intermediate, the π system involves only five of the carbon atoms.
In practice, this means that the electrophile normally needs to be a fully positive species that is formed as a result of the heterolytic fission of a covalent bond. An induced dipole within a molecule is not normally enough to achieve electrophilic attack in benzene.
It is not easy to form a sulficiently powerful electrophile to attack a benzene molecule. So a catalyst is used in the reaction mixture to generate the electrophile. Typical electrophiles that can be generated in this way include Br+, Cl+, R+, RCO+ and NO2+. (R is an aryl or alkyl group.) You will notice that the charge carried by the atoms involved is not often what you would have expected by considering their electronic arrangements, which offers an explanation for why catalysts are needed to generate the electrophiles.
HALOGENATION OF BENZENE
Chlorine and bromine can both be converted into electrophiles that will attack a benzene ring. The resulting substitution reaction makes chlorobenzene or bromobenzene.
C6H12 + X2→ C6H5Cl2 + HX (where X is Br or Cl)
Notice that the equation is written with molecular formulae rather than structural or displayed formulae. This is acceptable provided that there is only one structure possible.
Chlorination of benzene
When chlorine is bubbled through a mixture of refluxing benzene in the presence of a catalyst (such as aluminium chloride or iron(III) chloride), electrophilic substitution takes place to produce chlorobenzene:
C6H6 (l) + Cl2 (g) → C6H5Cl (l)+ HCl (g) (where FeCl3 reflux serves as the catalyst)
We already know the mechanism of this reaction: CI+ is represented by E+. The major question now is: how does aluminium chloride help to generate the electrophile?
We might expect aluminium chloride to be ionic, since it is a metal compound. However, in the anhydrous state it has a high degree of covalent character, so it is better when drawing a dot-and-cross diagram to show the electrons as shared. This structure has the molecular formula AICI3, though there is strong evidence to suggest that the formula is actually Al2Cl6.The dot-and-cross diagram will show that Al2Cl6, is an electron-deficient molecule, since the outer shell of the aluminium atom has only six elcctrons.
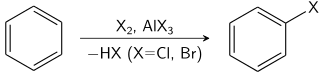
halogenation of benzene. Krishnavedala - Own work, Public Domain
Anhydrous aluminium chloride transfers its electron deficiency to a chlorine atom, to form the electrophile CI+ and a tetrachloroaluminate ion AICI4-. The catalyst is often referred to as a halogen carrier. Once the electrophile is generated, the reaction proceeds to give the substitution product. The proton eliminated by the benzene ring at the end of the reaction can then react with the AICI4- ion to give hydrogen chloride.
Anhydrous iron(III) chloride acts as a catalyst in the same way. In fact, iron filings could be used as a catalyst, because iron(III) chloride would be formed in the reaction vessel by the reaction between iron and chlorine.
Bromination of benzene
When a solution of bromine in benzene is refluxed with aluminium chloride, electrophilic substitution takes place to form bromobenzene:
C6H6 + Br2 → C6H5Br + HBr
Notice that the catalyst is not included in the overall chemical equation, even though it is intimately involved in the production of the electrophile.
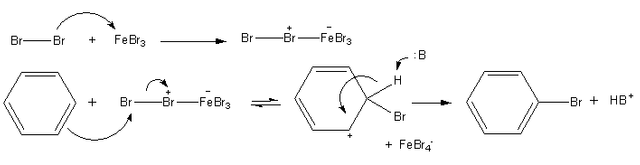
The mechanism for bromination of benzene. Jag123, Public Domain
We do not have to use aluminium bromide as the catalyst, because aluminium chloride will work instead, in which case the bromo-trichloroaluminate ion, AIBrCl3-, is formed instead of AIBr4-.
ALKYLATION OF BENZENE
This reaction is known as the Friedel-Crafts reaction. In it, a hydrogen atom is substituted by an alkyl group. The importance of this reaction is that a carbon-carbon bond is made. This time the problem is to make a carbocation that can act as an electrophile.
There are two main ways in which the electrophile (the carbocation) can generated. One involves the heterogeneous fission of a halogenoalkane, and the other is the electrophilic addition of a proton to an alkene.

Benzene ethylation. Krishnavedala - Own work, Public Domain
Carbocations from halogenoalkanes
Typically, benzene, a halogenoalkane and anhydrous aluminium chloride are refluxed together to give an alkylbenzene. For example, iodomethane gives methylbenzene:
C6H6 + CH3I → C6H5CH3 + HI (where AlCl3 refluxed serves as the catalyst)
Let’s look at the formation of the carbocation that permits this reaction. It shows that it is similar to the way in which the electrophile Cl+ is formed. Again, the aluminium chloride can transfer its electron deficieney, this time to an alkyl group, to form the carbocation.
Carbocations from alkenes
Alkenes typically react by electrophilic addition, When the electrophile is a proton, a carbocation is formed as an intermediate. When this is formed in the presence of benzene, the carbocation can react with Benzene, rather than completing the addition reaction.
Many different types of acid catalyst can be used in this reaction, including concentrated sulphuric acid, concentrated phosphoric(V) acid and aluminium chloride.
Manufacture of poly(phenylethene)
Poly(phenylethene), common name Polystyrene, is familiar to us as the material of hot drinks cups, ceiling tiles and packaging for fragile objects. Mobile telephones too are made from poly(phenylethene). It is manufactured from two readily available hydrocarbons, ethene and benzene.

Expanded polystyrene packaging. User:Acdx - Own work, CC BY-SA 3.0
Ethene reacts with benzene in the presence of aluminium chloride, with hydrochloric acid as an acid catalyst, to form ethylbenzene. This is an example of electrophilic substitution. The purpose of the acid catalyst is to generate an ethyl carbocation that acts as the electrophile.
The ethylbenzene from the first reaction provides the correct carbon skeleton for the next stage. This involves a dehydrogenation reaction, in which two hydrogen atoms are lost to form phenylethene. Finally, the phenylethene is polymerized normally under the influence of a free-radical initiator.
In my next post, I’ll discuss more about the acylation, nitration, sulfonation and the addition reactions of benzene.
Thanks for reading.
REFERENCES
https://www.chemguide.co.uk/organicprops/arenesmenu.html
https://www.scienceabc.com/humans/why-are-vehicles-exhaust-fumes-harmful-to-humans.html
https://en.wikipedia.org/wiki/Catalytic_reforming
https://en.wikipedia.org/wiki/Benzene
https://www.cs.mcgill.ca/~rwest/wikispeedia/wpcd/wp/b/Benzene.htm
https://en.wikipedia.org/wiki/Electrophilic_substitution
https://byjus.com/chemistry/electrophilic-substitution-of-benzene/
https://www.chemguide.co.uk/mechanisms/elsubmenu.html
https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/benzrx1.htm
https://en.wikipedia.org/wiki/Electrophilic_halogenation
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch23/ch23-2-1.html
https://www.chemguide.co.uk/mechanisms/elsub/halogenation.html
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This is a good effort Empress. I am not a chemist but I wasn't really bad at chemistry while in school, hence I understand a large part of this write up. I will really love to see chemistry written in a more "social" sense so as to make it more interesting for people without science background.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks, @gentleshaid. I'll surely work on that.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I am happy to see that you are back! This is a very good new for SteemSTEM. I hope life was smooth enough on your side during your absence.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Yeah, thanks @lemouth. I'm glad to be back. Life is full of ups and downs and smiles and frowns.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
As always :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit