What is atom?
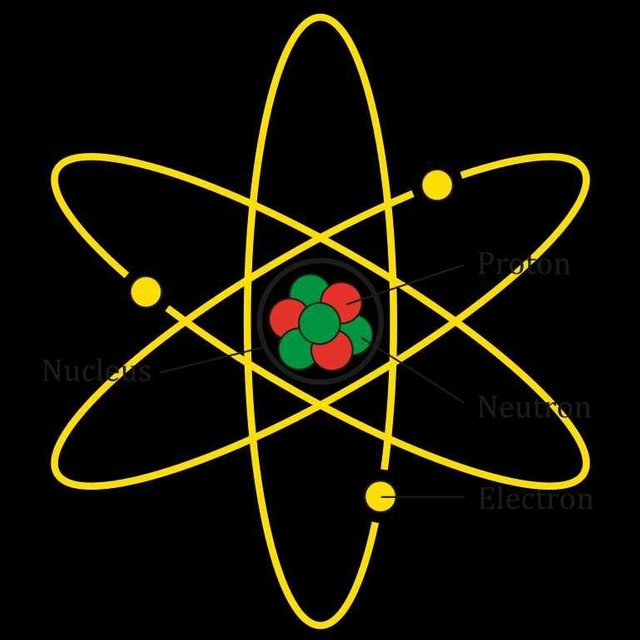
Image source
Licence
The smallest part of the chemical element, which does not have an independent existence (except for the inactive gas atoms), but the microscopic particles that can directly take part in chemical reaction are called atom. All the solid, liquid, gas and ion formations have the origin of the sediment or atrophy atoms. Atomic size is very small; Generally they are 100 pixels in length (10,000,000,000 parts in 1 meter or 1 in 1 million of hair).
Due to the small quantities of atoms, its behavior can not be explained by the formulas of traditional physics.
History of atomic theory
Indian philosopher Kanad gave the concept of atoms 600 years before the birth of Christ. He said that all materials are made up of small and uninterruptible particles. The Greek philosophers tried to create the theory of matter from philosophical perspectives but not with the use of experiments and observations. The first philosopher of this section was Leukippus of Miletus (5th century BC). His famous disciple, Abdare Democritus, named the "Atoms" (Greek: Atomos), whose literal meaning is "indivisible", which is named after the invisible particles formed by substance in 400 BC. Democritus believed that the atoms are balanced, strong, non-volatile and can not be destroyed. He believed that the size, shape, and layout of the atom are controlled by the religion of matter. As the atomic mass of the fluid is smooth, it is easily rolled over each other. But the atoms of solid materials are stuck with each other so that it is groovy and uneven. The rest of the material except atoms is only empty space. In democritus philosophy, atoms are not only confined to matter, it is also associated with the concept of human soul and sense. For example, he believed that the matter of the substance was formed due to the so-called atom and the white color is due to the smooth atom. The atoms of human soul were considered a very fine kind. Later, Samos' epicurus (341-270 BC) tried to remove the prejudice of ancient Greeks by using the philosophy of Democritus. He said that everything in the universe is made up of atoms and vacuums, so the Greek gods are not even above the natural law. Later, Plato and Aristotle opposed Democritus philosophy. Plato did not want to believe that the beauty and greatness of the materialistic atom is the mechanical expression. And Aristotle rejects the idea of vacuuming because he could not imagine that different objects would go through the same void in the same speed. This idea of Aristotle influenced Medieval Christians in Europe. Roman Catholic priests reject democritus philosophy as materialists and atheists.
Structure of atom
Scientists have done a lot of research on how the structure of the atom could be. One of these is Dalton's Paranoidism. The foundations of modern chemistry are known to have been found to be atomic in atoms in Dalton. But this theory is now dead. At the end of the nineteenth century it was proved that three elements of the atom. The forming particles that form atoms with particles are called basic particles. They are electrons, protons and neutrons. These three particles consolidate into different numbers and form different atoms. The positive-charged proton and the non-particle neutron together form the nucleus and surround them with the negative charge electrons moving around.
Electron: The smallest particle electron in atom. In 1897, Sir J. J. Thomson discovered the existence of the first electron. The real mass of an electron is very little 9.1085 × 10-28g. Electron inlay -1.6 × 10-19 Coulomb Rotating around the electron nucleus. The electron is usually released by e symbol.
Proton: Proton is a positive particle that is contained in the nucleus. In 1911, the existence of scientist Ernest Rutherford proton proves that an atom has an equal number of protons. Proton mass is 1.673 × 10-24g, which is 1.007276 amu (amu here atomic mass unit) according to the nuclear mass scale. Proton is available when an electron is removed from a hydrogen atom, so it can be called H +.
Neutron: Neutron, like electrons and protons, is also a basic particle but it is unstable. It is named because it is neutral. The neutron is located in the nucleus. In 1932, James Saddwick discovered the neutron. Its original mass is 1.675 × 10-24g which is 1.008665 amu according to the nuclear mass scale. Its mass is about 1837 times the mass of electrons. It is usually expressed by n. The neutron is connected to the proton in the center of the atom. The combined mass of these two particles is called a nuclear mass.
Atomic
- Dalton's atomic: In 1803, English physicist and chemistry scientist John Dalton published a theory about atoms which are known as Dalton's atomism. There are five statutes in his given atomic power. These five are:
The substance is made up of very small particles, the names of these particles are atoms.
The size, mass, and other properties of atoms of the same substance are the same, the size, mass, and other properties of different substances differ.
Atoms can not be divided, created or destroyed.
In simple total numbers, the atom of different steps can be combined into chemical compounds.
Atomic amplification, split or re-formulated in chemical sales.
Until the middle of the 18th century, scientists accepted Dalton's admission Because they did not have enough information to deny the statements. In 1897 JJ Thomson discovered the electron. As a result, parts of Dalton's third account (can not be divided atom) prove to be false.
Thomson atom: With the 1898 scientist JJ Thomson proposed this model. According to this model, electrons spread throughout the pudding, such as kisemis, sporadically spreading, at the same time, the electrons circulate in a continuous distribution of atomic particles.
Rutherford's atomic: In 1909, scientist Ernest Rutherford performed alpha particle distortion test. In 1911, he came to the conclusion that the atom has a very heavy element of positive charge in the center of the atom. It was named after the Nuclearists. Elements of electrons and the rest of the atoms are rotating around the nuclei.
Nile Bore's atomic: In 1913, Danish physicist Nellis Bohr made two proposals for his nuclear model, which is known as Boore's term.
Postulates of Stationary States
Frequency of Postulates
He said that electrons are rotating on some approved paths around the nucleus, and he is the first to give an idea about spectrum.
Electrons in atoms
The exact number of electrons in an atom is equipped with certain structures in the atomic masses of the atom, which are arranged in an orbit of specific substrates; this is called the electron structure of the atom's electron form. The electron formula can be explained by rules of policy, by the rules of aufbau's and the rules of the hound.
Abolition Policy: The value of four quantum numbers of two electrons (usually two pharmacy's) can not be the same at an atom. At least one of the values is different from the two electrons.
Aufbau policy: Atoms of electrons in atoms will take position at the lowest energy level at the time of various occupation, after the lower power level is completed, it will move to the next higher energy level.
Hound formula: The electrons in the same orbital arithmetic enter in such a way that they may remain in maximum or odd conditions, the spin or spin of the ephemeral electrons will be the same. Here are three orbital orbital orbital orbital orbital orbital orbital orbital orbital orbitals Note that the sources of the hound for s orbitals are not propose.
Reference
- What is an atom? Web Document
- Atomic, Web Document
- A Brief History Of Atomic Theory, Web Document
- Structure of the Atom, Web Document
Thank you for read this post.If you have any question about science, ask in the comment box.I will try to give answer your question.Follow me to get more information about science.
Join my discord server.
Follow me on Facebook
Follow me on Linked In
Follow me on Twitter
Follow me on Google+
If you are interested in science, technology and mathmetical things on steemit, join the steemstem community. To get more informtion about steemStem click here. Join steemStem discord server.

Source
Direct translation without giving credit to the original author is Plagiarism.
Repeated plagiarism is considered spam. Spam is discouraged by the community and may result in action from the cheetah bot.
More information on Image Plagiarism
If you believe this comment is in error, please contact us in #disputes on Discord
Please note that direct translations including attribution or source with no original content is also considered spam.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations!
This post has been upvoted from Steemit Bangladesh, @steemitbd. It's the first steemit community project run by Bangladeshi steemians to empower youths from Bangladesh through STEEM blockchain. If you are from Bangladesh and looking for community support, Join Steemit Bangladesh Discord Server.
If you would like to delegate to the Steemit Bangladesh, you can do so by clicking on the following links:
50 SP, 100 SP, 250 SP, 500 SP, 1000 SP.
YOU ARE INVITED TO JOIN THE SERVER!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks for using eSteem!
Your post has been voted as a part of eSteem encouragement program. Keep up the good work! Install Android, iOS Mobile app or Windows, Mac, Linux Surfer app, if you haven't already!
Learn more: https://esteem.app
Join our discord: https://discord.gg/8eHupPq
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @mr-science! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPDownvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hi @mr-science, your post has been upvoted by @bdcommunity and the trail!
If you want to support us, please consider following our curation trail, setting us as your witness proxy, or delegating STEEM POWER to us.
JOIN US ON
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by mr-science from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This post received a 50% UpVote from @TechMojo.
Promote your work on the TechMojo Discord Channel
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit