The strongest acid in the world Carborane acid is carboric acid, which is by far the strongest compound in the world. The formula of the strongest acid looks like this: H (CHB11Cl11). This monster was created in 2005 at the University of California, in close cooperation with the Novosibirsk Institute of Catalysis of the SB RAS. The very idea of synthesis arose in the minds of scientists, along with the dream of new molecules and atoms that had never before been seen. The new acid is a million times stronger than sulfuric acid, while it is completely non-aggressive, and the strongest acid can easily be stored in a glass bottle. True, over time, the glass still dissolves, and as the temperature rises, the rate of such a reaction increases significantly.
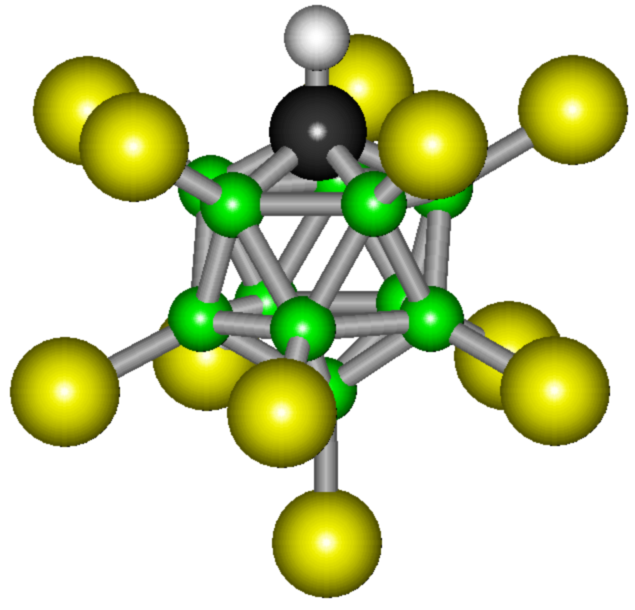
Such surprising softness is caused by high stability of a new connection. Like all chemicals related to acids, carborane acid easily reacts by giving away its only proton. At the same time, the acid base is so stable that the chemical reaction does not proceed any further. Chemical Properties of Carboric Acid The new acid is an excellent donor of the proton H +. This is what determines the strength of this substance. The solution of carboric acid contains more hydrogen ions than any other acid in the world. In the chemical reaction, SbF5 is antimony pentafluoride, binds the fluorine ion. In this case, new and new hydrogen atoms are released. Therefore, carborane acid is the strongest in the world - a suspension of protons in its solution is more than a similar indicator of sulfuric acid in 2 × 1019 times. However, the acid base of this compound is stunningly stable. The molecule of this substance consists of eleven bromine atoms and the same number of chlorine atoms. In space, these particles form a complex, geometrically correct figure, which is called an icosahedron. This arrangement of atoms is the most stable, and this explains the stability of carboric acid.
The value of carboric acid The strongest acid in the world has brought its creators well-deserved awards and recognition in the scientific world. Although all the properties of the new substance have not been fully studied, it is already becoming clear that the significance of this discovery goes beyond the scope of laboratories and research institutes. Carborane acid can be used as a powerful catalyst for various industrial reactions. In addition, the new acid can interact with the most stubborn chemical substances - inert gases. At present, work is under way allowing xenon to react. Undoubtedly, the amazing properties of new acids will find their application in the most diverse fields of science and technology.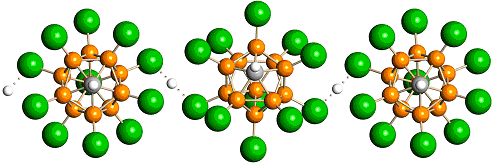
Subscribe to my blog, because you will find there a lot of interesting things