Hello dear Artzonians!, this afternoon as a teacher of Junior High, I want to teach separation of mixture, thus, separating alcohol from water using Simple Distillation method. I have to improvise since I do not have the real apparatus to carry out the experiment.
I got my cardboard, and permanent marker and started drawing the experimental set-up for separating alcohol from water using Simple Distillation Method.
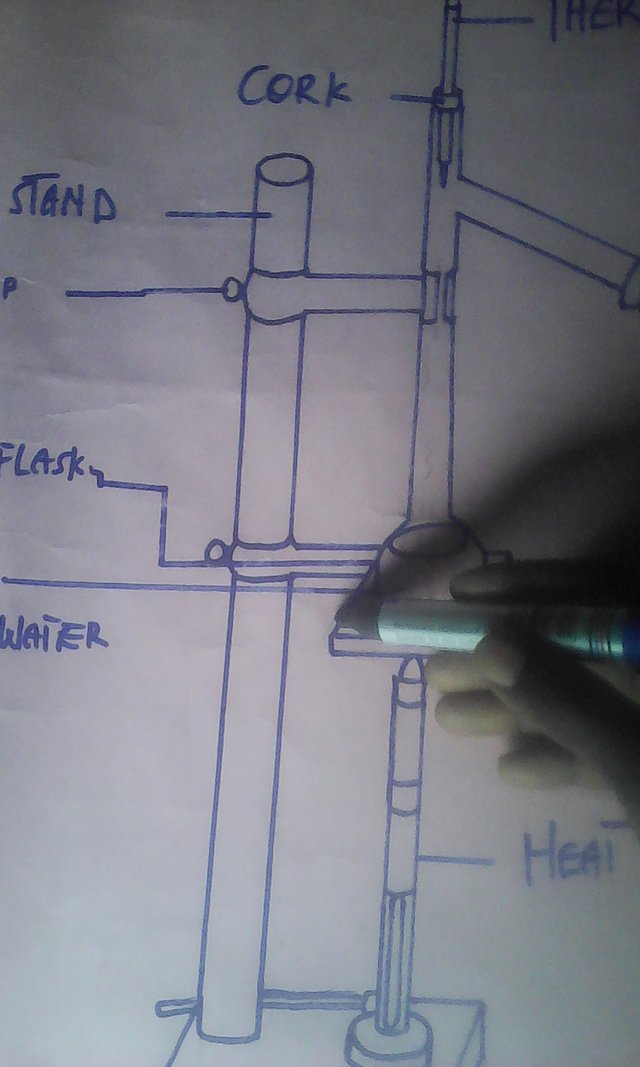
The set-up illustrate a distillation flask which contain palm wine stored for several days mounted on a source of heat. Thermometer is placed at the neck of the distillation flask well tied, in order to check the temperature levels of the original alcohol and water. Since alcohol boils at 78 degress Celsius, and water at 100 degrees Celsius, the alcohol will evaporate first, leaving the water behind.
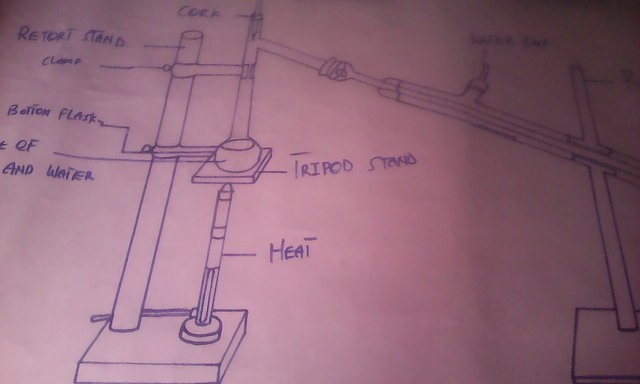
This will occur for several times until all the alcohol becomes exhausted, then the water is allowed to come out from the outlet on the set-up. The condenser on the experimental set-up serves as a cooler region to cool up the evaporated alcohol into liquid nature. The distillated is collected in another container, till it ceases to come.
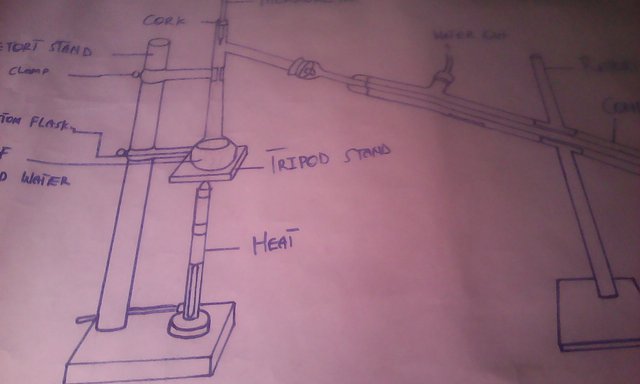
This is how the method is carried out to separate alcohol from water, that is having two different substances with different temperature levels. You can also add your opinion to mine how this method can also be done in a different way. Thank you for considering my sketch for this experiment.