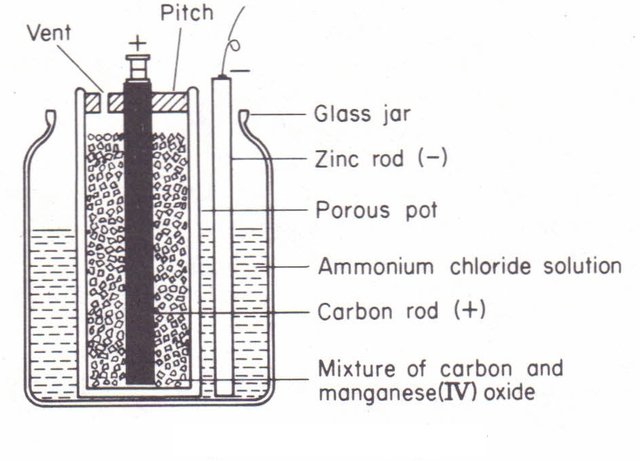
Leclanché Cell was invented by Frances Scientist Georges Leclanché in 1866.[1] [pdf] There is a glass container which is filled with ammonium chloride. in the solution, a rod of mercury-coated zinc is submerged which acts as a negative pole. a jar containing foramen made of ceramic is dipped in solution, in which the carbon rod is kept inside. which works in positive duality. in this jar, a mixture of manganese dioxide and granulated carbon is filling.[2] manganese dioxide acts as a depolarizer and transmits granular carbon electrons to ammonium ions.
When the carbon and zinc rod is added to the circuit then in contact with electrolytes, zinc atoms are ionized by giving two electrons.
Zn → Zn2+ + 2e-
Ammonium chloride (NH4Cl) in the electrolyte is thus ionized:
2NH4Cl → 2NH4+ + 2Cl-
Zinc ions make zinc chloride combined with chlorine ions.
Zn2+ + 2Cl- → ZnCl3
Ammonium ions (NH4+) flow towards the carbon rod and become Indifferent by taking an electron from the carbon rod, ammonium is converted into hydrogen gas.
2NH4+ + 2e- → 2NH3 + H2
Manganese dioxide converts hydrogen gas into water, which does not cause the action of polarization.
2MnO2 + H2 → Mn2O3 + H2O
Full Reaction
Zn + 2NH4Cl + 2MnO2 → ZnCl2 + 2NH3 + Mn2O3 + H2O + Energy [3]
Thus, the excess of electrons on the zinc rod and the lack of electrons on the carbon rod decreases, whereby the potentiality of carbon rod becomes more than zinc rod potential. So the electrons start flowing towards the carbon rod from zinc rod. The Electromotive force of the cell is 1.5 volt.
Manganese dioxide is in the solid state. It can not quickly convert hydrogen into water. Some hydrogen gas starts to accumulate in the carbon rod, resulting in partial polarity and the electric current becomes dim. but if the cell is rested, the hydrogen frozen on the carbon rod becomes oxidized in water. due to this defect, this cell is used in the telephone, telegram, electric bell.[4]
PDF Source: "The Leclanché Cell. Museum Notes, Oesper Collections"
Reference:
- Scientist Georges Leclanché
- Zinc-Carbon Batteries, Molecular Expressions
- Commercial galvanic cells: Leclanché Dry Cell
- Battery Facts