TLDR:
- the cigarette filter was invented to extinguish cancer fears
- 5 trillion are consumed yearly making them the most littered item in the world
- in addition to an astonishing amount of additive, filters trap mostly carcinogenic tar
- ingestion is toxic to children and pets and as little as 1 butt per litre of water can kill fish, worms
- filters are mostly cellulose acetate, a polymer that breaks down into microfibres after a decade
- microplastic attracts similarly lipid-based pollutants which adsorb to its surface
- plastic is then ingested releasing toxins into system of host which 'bioaccumulate' in fat stores
- this contamination can be 'biomagnified' up the food chain eventually to fish consumed by humans
- responsibility is with consumer, to get informed and provide good feedback
Chemical engineering
Tobacco companies first began developing filters for cigarettes in the early 1950s as concerns emerged linking the product with lung cancer. Phillip Morris, British-American Tobacco and others hired chemists at DuPont and Dow Chemical to engineer the filter. After a few setbacks, involving one from Kent found later to contain asbestos, a design was finalised and has remained in use since: the cellulose acetate filter. Another chemist named Claude Teague was able to add a feature by adjusting the pH conditions in the filter causing it to turn brown upon use, giving the impression of catching harmful substances (Figure 1).

Unfortunately, as it turns out now, filters provide little to no protective benefit, and may have caused more harm than good - making it easier for the wider community to take up the habit. By improving the safety image and reducing harshness into the respiratory tract, filtered cigarettes allowed tobacco companies to expand their market share to a younger more female demographic. And there is a growing literature showing since its addition, the filter has contributed to the rise in cases of lung adenocarcinomas, as users tend to compensate for the lower nicotine dose by drawing more smoke deeper into the lungs.
End of life-cycle
As well as being responsible for the deaths of an estimated 6 million people per year, cigarettes are also the most littered item in the world. Globally, 6.3 trillion cigarettes are consumed per year with China alone making up one third of this market. Due to their lack of biodegradability in design, cigarette filters or ‘butts’ have become one of the biggest contaminants of our oceans, frequently found across coastlines from Asia to the Arctic with butts being the most common item of trash collected by Clean up Australia Day for the past twenty years in a row.
There are two main endpoints in the life cycle of a cigarette:
If consumer does the right thing and throws it in the bin, the cigarette butt will end up in landfill. Potentially contributing to the emerging problem of microplastics in soils or escaping out into the surrounding environment.
The second option is the more scrutinised littering. Meaning in most cases, discarded on the street down drains and transported via waterways before deposition into lakes wetlands and/or the sea.
Just as an example, in Australia an estimated one in every three of the 24 billion cigarettes sold annually becomes litter (Figure 2).

What is the cigarette filter made from?
The filters themselves are a fibrous web of micro-thin plastic. These fibres are composed of cellulose acetate (CA), a polymer synthesised by mixing cellulose (from cotton or wood pulp) with acetic acid. To make a uniform structure, the CA is then dissolved in acetone and spun into a product called tow. The largest producers of cigarette filter tow today are Eastman Chemical (formerly Eastman Kodak) and the recently merged Celanese and Blackstone.
When it comes to the many chemicals associated with cigarette filters, there are two main categories; compounds that are added during manufacturing, and those it then catches as the cigarette is smoked:
Additives
Triacetin is introduced to the CA-mix as a plasticiser making the fibres more soft and durable. Triethyleneglycol diacetate (TEGDA) can be used instead, with plasticiser making up around 6-12% the weight of the filter. Ethylene glycol and polyvinyl alcohol are next on the list as a copolymer and bonding agent, mainly to catch phenols. Flavourings (usually menthol) can also be mixed in with the plasticiser or infused with cotton thread.
Carbonates and potassium salts are added to control the burn rate around the paper, and titanium dioxide whitens the cellulose fibres. As well as dyes, flame retardants and glues (poly and ethylene vinyl acetates, PVA and EVA) for the tip (Figure 3), paraffin oils and even trace amounts of Bisphenol A can be introduced along the process.
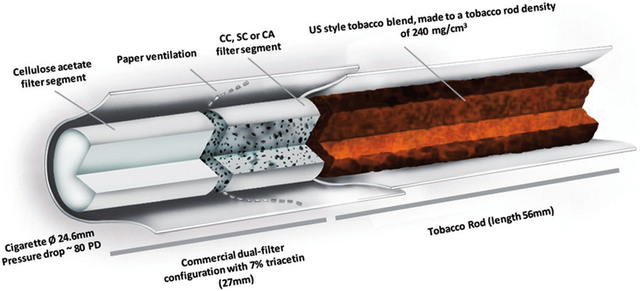
Considering the main role of the filter is to catch carcinogens released when tobacco is burnt, humectants (glycerol and propylene glycol) are applied along the entire 'rod' of the cigarette (Figure 4). By increasing the water retention factor of the CA fibres, humectants keep the filter moist and improve its 'wicking' ability trapping more tar and smoke.
Tar & smoke
Burning or pyrolysis of tobacco releases a concoction of gasses and particulate (in solid and liquid form) containing over 5000 chemicals. At least 70 of which are classed as cancer-causing by the World Health Organisation. Unfortunately for smokers, filters are mostly ineffective at stopping the gas constituent; hydrogen cyanide, nitrogen and carbon oxides for example mostly pass straight through.
A significant fraction of the liquid and solid tobacco end-products, however, are trapped in the filter thanks to its design, as resinous tar and smoke particles have a relatively high affinity for the moistened fibrous surface area within the CA-matrix.
Such compounds include:
Metals, arsenic, lead, chromium and cadmium. Long-term or chronic exposures of each have been linked to a number of lethal outcomes including heart disease and cancer, with trace amounts negatively affecting most organs in the body.
At least a dozen variants of a family compounds known as polycyclic aromatic hydrocarbons or PAHs, all of which have been found to promote mutations, abnormal development (teratogenic) and proliferation of cancer cells in both animal and in vitro studies.
Butadiene, benzene and ethylene oxide, each assigned Group 1 status - the highest level of cancer-risk to humans by the International Agency for Research on Cancer (IARC 2018) and all associated with cancers of the blood and bone marrow such as acute myeloid leukemia and non-hodgkin's lymphoma
Naphthylamine and aminobiphenyl both aromatic amines and Group 1 carcinogens, linked to mutations in liver tissues and cancer of the bladder.
Tobacco-specific nitrosamines (TSNAs) such as N-nitrosonornicotine (NNN) and nicotine-derived nitrosamine ketone (NNK) recently discovered highly carcinogenic nicotine by-products, that persist long after the cigarette smoke has dissipated.
A veritable carcinogenic alphabet soup ensues, including ammonia, acrolein, acrylamide, formaldehyde, phenols (cresols, catechol, 4-ethylpehnol), pyridines, paraffins and terpenes, volatile organic hydrocarbons(acetaldehyde, isoprenes), uraniumand the radioactive isotope polonium-210.
Secret ingredients
Making a comprehensive list of all the ingredients and chemicals associated with cigarette filters is complicated. This is compounded by the fact that because tobacco is not regulated as a food or drug, undisclosed substances can be added, either as propriety ingredients or inadvertently due to contamination somewhere along the supply chain. These can include any number of flavourings(sugar/sorbitol, liquorice, cocoa) pigments, preservatives, adhesives, fumigants and even byproducts of the agricultural and manufacturing process such as fungicides, insecticides, metals, fuels (propane, pentane) and other impurities such as the banned CFC freon gas.
The icing on the cake is a dizzying array of other patented fragrance promoters, masks, 'performance enhancers' and irritation-reducing agents with names like 'Aromatek 245', 'XLF 680' and 'Studio 26 blend'. This aromatic cocktail is mixed with alcohol and sprayed onto the reconstituted tobacco, making up around 1-1.5% of the 'recon' (Figure 5).

In all, total non-tobacco product has been estimated to comprise as much as one-tenth the mass of a cigarette.
Pyrolysis products
Little is known as to how all these chemicals might react when exposed to extreme temperatures within the cigarette (over 900 degrees celsius) or as the burning tip comes into close proximity with the filter (Figure 6 below). For example, there are concerns that triacetin, which makes up a large proportion of the filter, could be “eluted in the smoke during the last few puffs” due to its “relatively low boiling point (258°C)”, though it's important to note the manufacturers are more concerned with the bitter taste than they are any implications of the chemical changes taking place with pyrolysis.

Determining the products of any combustion reaction can be unpredictable. Glycerol, one of the humectants added to tobacco, dehydrate to acrolein - a known environmental contaminant found to inhibit phagocytosis and mucosal clearing of the lungs. And the formulation of dioxins, often produced as a result of burning synthetic chemicals such as glues and plastics, is also a concern - dioxins have been placed on the ‘dirty dozen’ list as some of the most persistent and carcinogenic substances known to man.
Measured levels
Unsurprisingly, a number of these aforementioned chemicals, particularly metals, have be measured at relatively high levels in discarded and used cigarette butts (Figure 7 below). Making them a likely point source for pollution. In 2009 for example, researchers Moriwaki and others found cigarette butts along roadsides in Japan contaminating the surrounding environment with leachate containing PAHs and metals including arsenic, lead, copper, chromium and cadmium. And Dobaradaran and others also detected considerable metal concentrations within filters discarded around the Gulf of Persia. Moerman & Potts backed this up in the lab placing smoked filters in solution and finding increased concentrations of all 12 trace metals tested (except cadmium) after just one day of immersion.
Toxicity
Effects of exposure to cigarette leachate (the solution resulting from cigarettes in water) are well studied (Figure 8). Slaughter et al 2011 for example found an EC50 (effective concentration) of 1 filter/litre. In other words, one used cigarette filter per litre of water was enough to kill half the populations in both samples of fish (Atherinops affinis and flathead minnow) within 48 hours. Frog (Xenopus laevis) embryos were susceptible at similar concentrations. And Misceva et al 2006 observed an EC50 in water fleas as low as 26 milligrams of filter per litre; nicotine and 4-ethylphenol among the suspected active agents. Mortal (and sub-lethal) outcomes induced by cigarette-leachate have been documented across a range of species groups, including snails, fish, mosquito larvae, worms and even plants.

The potency of cigarette butts to humans, specifically infants, is also well known. In 1996 for example the Centers for Disease Control and Prevention in the US reported almost 8000 cases of cigarette butt ingestion with poisoning brought on by the acute dose of nicotine, not to mention the cocktail of accompanying tobacco byproducts. A single butt contains about 5 milligrams of nicotine doses as low as 1 mg can induce high blood pressure nausea and life threatening seizures in small children. And pets are not immune either, exhibiting similar susceptibility with cases of ingestion documented in both dogs and birds.
Unfortunately adequate information regarding ingestion of cigarette butts in the wild is scarse meaning at this stage, little is known as to how much a threat cigarettes and associated debris pose, both to individuals and the larger ecology as a whole (Figure 9). Though, considering cellulose acetate is a plastic, concerns are certainly valid considering the growing list of negative impacts being attributed to microplastics.

Filter fibres
Due to the strength of the ester bonds in the cellulose acetate polymer, cigarette filters are not readily biodegradable (i.e. cannot be eaten by microorganisms). They are however degradable, meaning each filter (a complex of over 12,000 plastic fibres) breaks down in the environment after around 12-15 years with exposure to heat and light (photo-degradation).
The organic (carbon-based) structure of cellulose acetate could mean the fibres (like other plastic debris) act as a magnet for similarly-soluble organic molecules. It is this high affinity for plastics that allows a wide variety of hydrophobic (water insoluble) contaminants, already present in the environment, to stick and build up on the large surface area produced as plastic breaks down. Such adsorbents often include any number of synthetically derived, industrial, pharmaceutical, and/or agricultural chemicals.
POPs
Most notable among environmental pollutants are the persistent organic ones, also known as POPs. A glance at the full list of POPs established by the Stockholm Convention reveals some fairly well-known groups; the polychlorinated insecticides and solvents such as DDT (dichlordiphenyltrichlormethane) and PCBs (polychlorinated biphenyls), the brominated biphenyl and diphenyl ether flame retardants, PBBs and PBDE respectively, and the more recently listed flourinated surfactants - perfluorooctane sulfonic acid (PFOS) and sulfonyl fluoride (PFOSF).
Though most of the original POPs have now been banned, their longevity allows them to cover vast distances and remain prevalent in the environment to this day. DDT and dieldrin for example, in addition to showing up in human blood and breastmilk, have been detected in soil up to and even over a decade post-application. And even though they were banned in the 70s, PCBs are still being found in the Great Lakes off Michigan.
A great deal of other chemicals, though not officially listed as POPs, are still of concern including many brought in as POP-replacements; i.e. the nitrogen-based (atrazine) and carbamate insecticides developed to replace organochlorines, the organophosphate flame retardants to replace the brominated ones, and the latest PFOS-alternative, PFOA (perfluorooctanoic acid) which is being found almost as persistent as its predecessor.
Because of their carbon-based structure, many of these chemicals are lipid-soluble and therefore attracted to the similarly lipophilic surfaces of oil-byproducts such as plastic (Figure 10).
Microplastics
Mistaken as food or inadvertently ingested, microplastics are frequently found in the stomach, intestines and other tissues of wildlife, including marine mammals, birds, fish, crustaceans and plankton.
Once inside an organism a piece of microplastic can induce a stress response either at the ingestion site by physical abrasion, or circulating around body potentially causing chronic inflammation to tissues and organs.
More subversively, a plastic particle can also act like a pill, releasing biologically active chemicals (either manufactured into its polymeric structure or sorbed onto its surface from the surrounding environment) into the blood supply of its host.
The intrinsic (built-in) chemicals can include many plasticisers and additives such as phthalates and bisphenol A, flame retardants (PBDE), and even the antibacterial agent, triclosan; all of which have been found to escape from their respective polymer chains and interfere with hormone receptors thus disrupting the endocrine system.
The extrinsic (adsorbed) chemicals are mostly non-polar with an affinity for the oil-based surface of plastic. This includes many of the aforementioned POPs that enter the sea from industrial, domestic and agricultural sources. PCBs, dioxins, fluorinated surfactants, chlorinated pesticides and even pharmaceuticals from wastewater have all been found associated with plastic debris.
If microplastics are vector or conduit by which such toxicants gain entry into living systems, then cigarette filters, whole or in part, could be acting similarly. And though I wasn’t able to find studies confirming if POPs have been found associated with cigarette-derived cellulose acetate specifically; one might assume being a carbon-based polymer, CA would exhibit similar affinity for organic contaminants.
So in addition to carcinogenic tar, if filters are trapping environmental contaminants as well, this would further increase their toxicity. And considering cigarette butts are often mistaken for food and have apparently been found in fish, turtles and birds. This would make butts a potential delivery system not only for the host of tobacco-associated pollutants but any additional adsorbed ones as well.
Bioavailability and accumulation
After desorbing from the plastic particle in the low pH conditions of the stomach, a bioavailable chemical, being lipid-soluble, can freely cross the gut membrane and enter systemic circulation. If bioactive, then enact changes to enzymes in target tissues, and if large enough to overcome homeostasis - then affecting organs such as the liver. Otherwise another pathway is bioaccumulation usually in adipose tissue(Figure 11).
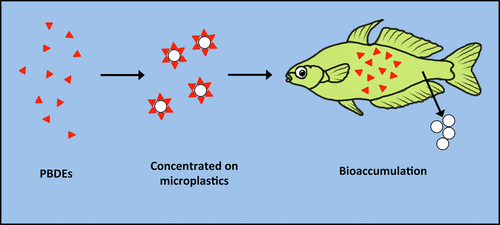
One of the most immediate threats, particularly from a human health standpoint, is then the transfer of this accumulation up the food chain.
Biomagnification and dinner
Biomagnification occurs due to predator-prey dynamics within the hierarchy of marine life, whereby any exposures to contaminants lower down tend to exponentially increase up to the point where higher order predators, because they consume such a large number of species, tend to have higher concentrations of these substances in their tissues.
To put it simply, the accumulation is 'magnified' up the food chain (Figure 12). This is essentially a 'Russian doll' effect, or to quote Star Wars, "there's a bigger fish".
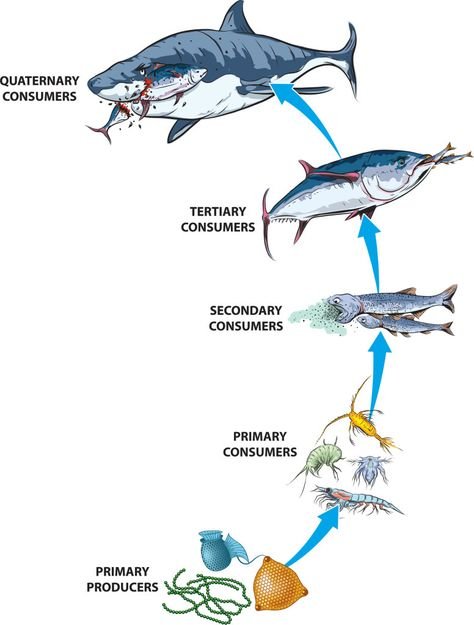
Particles of plastic have been found digestive tract, livers, gills and other tissues of fish and shellfish commonly consumed by humans (Figure 13). The full list includes many species of cod, bream, bass, flathead, flounder, mullett, hake, swordfish, snapper, whiting, herring, anchovy, pilchard, mackerel, shark, tuna, salmon and shellfish (crustaceans, oysters, mussels, shrimp).

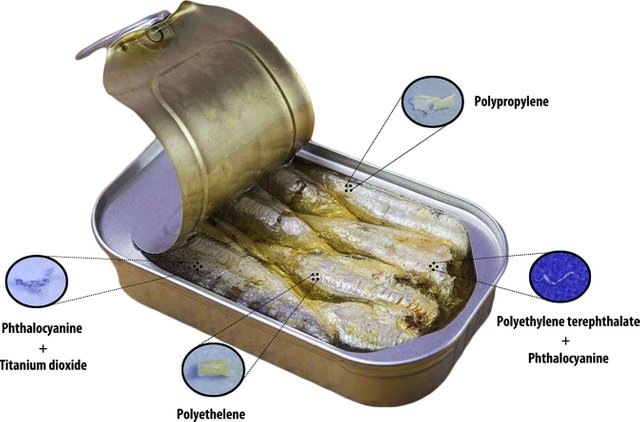
last year researchers Karami et al 2018 tested 20 brands of canned fish (sardines and sprats) and found 4 brands contained between 1-3 particles (mostly PET and PP) per can (Figure 14).
Even human stool is now being tested, researchers found all 8 subjects tested positive for micro plastics averaging 20 particles per stool.
Sadly there are even growing concerns that health benefits of sea food, specifically in regards to omega 3 fatty acids in products such krill oil, could become negated by the amount of contamination these creatures are exposed to.
What to do
In summary, the responsibility lies with the consumer, though not in the way we are commonly told..

It is purely my opinion, but NOT littering isn't solving much - it's just slowing the problem down.

The responsibility I'm talking about is our responsibility I believe we have as both voters and consumers, that if we notice a problem, to
a. inform ourselves as best we can so that
b. we may be better able to provide helpful, implementable feedback to the relevant corporations and our parties, or maybe even
c. come up with a solution ourselves.
So I've provided the contacts for some of the corporations mentioned in this article, if you wanted to ask what they are doing about the problems and if they are developing a better design.
Celanese @celanese
Eastman Chemical @EastmanChemCo
The Australian Government could implement a strategy that recognises cigarette butts as hazardous waste similar to how we treat batteries.
I recommend getting in touch with your local representative on this issue
So far only [one party]((https://www.ldp.org.au/legalisevaping) is on board the development of safer and less environmentally damaging (albeit illegal in this country) alternative to traditional cigarettes .
I've also added some of the major parties contacts if you wanted ask why they aren't:
ALP Twitter: @AustralianLabor
Liberal party](https://www.liberal.org.au/contact) @LiberalAus
Greens @Greens