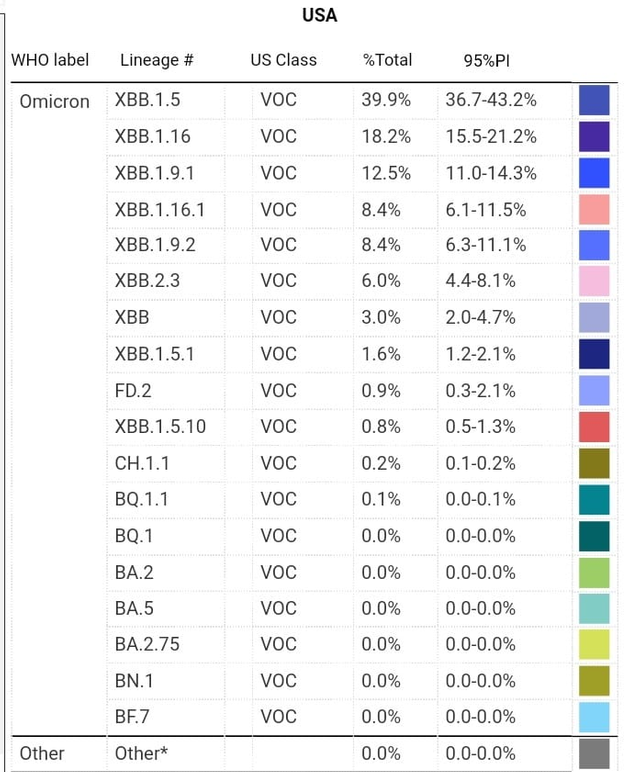
Expectations are for it to be a monovalent update of one of the XBB variants. They represent nearly all new cases in the US these days. Particularly XBB.1.5 and XBB.1.16.
The decision is relatively straightforward compared to the earlier strain composition update as these variants have quite similar spike proteins and have similar levels of immune response. The WHO's advisory committee previously last month recommended a monovalent update to XBB.1.5. While generally the FDA matches WHO on strain composition choices, they notably went with BA.5 while the WHO went with BA.1 for the previous Omicron boosters.
Since global immunity is so high, experts believe a bivalent vaccine is no longer necessary, so a shift to monovalent vaccines is expected.
The FDA's advisory committee VRBPAC voted unanimously to move to a monovalent XBB-lineage coronavirus vaccine.
They won't vote on the specific strain update, but they generally leaned towards XBB.1.5. The strain update is fairly simple this time as the circulating variants have fairly similar spike proteins. These updated vaccines are expected by September according to the FDA.