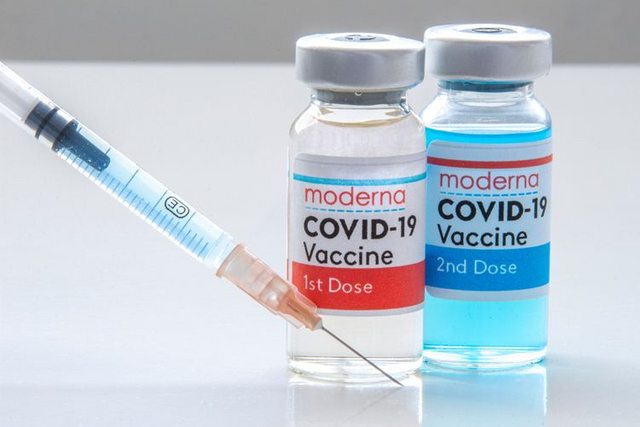
Three new conditions that a small number of people have reported after vaccination with COVID-19 injections from Pfizer and Moderna are being studied to assess whether they may be side effects. This was disclosed by European drug regulators.
Erythema multiforme is a form of allergic skin reaction, glomerulonephritis, or inflammation of the kidneys; and nephrotic syndrome, a kidney disorder characterized by severe urinary protein loss, is being studied by the European Medicines Agency (EMA) safety committee, according to regulators.
The success of the mRNA technology used by both vaccines has been a turning point for the pandemic and the scientific community, but some rare side effects are being studied as more people are inoculated globally.
Pfizer, which is by far the largest supplier of a COVID-19 vaccine to the European Union, and Moderna were not immediately available for comment on the new cases. More than 43.5 million doses of Moderna's vaccine, Spikevax, had been administered in the European Economic Area as of July 29, compared to more than 330 million doses of Pfizer's vaccine, Comirnaty developed with the German vaccine, BioNTech.
The watchdog gave no details on Wednesday as to how many cases of the new condition had been recorded, but said it had requested more data from the company to study a possible link between them.
The EMA itself makes no recommendations to change vaccine labeling. It reveals the new assessment as part of a regular update to the safety section of all official vaccine databases, and adds menstrual disorders as another condition being studied for all vaccines, including with AstraZeneca and J&J, following last week's EMA update.