Why can not you Build your own Biosensor
Development of a Biosensor for glucose detection
The actual electrode of model DS 110. The black circle in the middle, called the working electrode. Glucose oxidase and prussian blue will adhered to it via the method of amperometry. We will also use a subclass of amperometry, CV, cyclic voltage, and after designing it, we can apply beverages or fluid samples on the electrode to measure sugar content.
Background
I had some time over the other day, and some glucose oxidase to spare so i decided to design 4 x glucose biosensors for fun, since I had all the needed ingredients. I pulled out an old recipe and design schedule, and made a 1st generation glucose sensor from scratch on a DS 110 carbon diode electrode with an Ag/AgCl counter electrode. The sensor is based on Prussian blue, glucose oxidase and some usage of cyclic voltammetry/amperometry. I chose a cheap and easy to replicate design so you can try it yourself.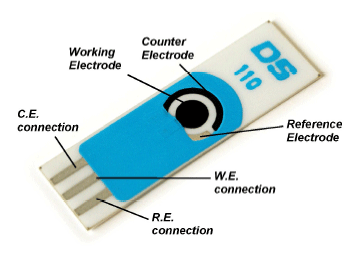
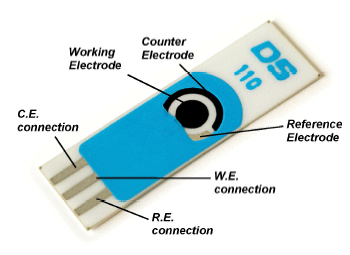
The actual electrode of model DS 110. The black circle in the middle, called the working electrode. Glucose oxidase and prussian blue will adhered to it via the method of amperometry. We will also use a subclass of amperometry, CV, cyclic voltage, and after designing it, we can apply beverages or fluid samples on the electrode to measure sugar content.
Background
I had some time over the other day, and some glucose oxidase to spare so i decided to design 4 x glucose biosensors for fun, since I had all the needed ingredients. I pulled out an old recipe and design schedule, and made a 1st generation glucose sensor from scratch on a DS 110 carbon diode electrode with an Ag/AgCl counter electrode. The sensor is based on Prussian blue, glucose oxidase and some usage of cyclic voltammetry/amperometry. I chose a cheap and easy to replicate design so you can try it yourself.

Schematic image showing differences in the generations of biosensors. I used a design from the 1st generation and added an catalytic element, since it covers a lot more chemistry and some engineering as well as is it being the easiest to rerpduce
I will provide the full list of ingredients and a detailed design plan that anyone can follow!
p.s all the images and facts with no references are my own design and I give full rite of usage to everyone in any context.
I measured the sugar content in what I could get my hands on around the laboratory, which was coffee, coffee with sugar, soda, diluted soda and some PBS(who doesn't have that doh)
The first design worked well and here is the data from my trail run. It took me about 4 hours to set up 4 x sensors due to using cyclic voltammetry and the time it requires.
What is biosensors, and what is the glucose sensor used for?
The definition of a biosensor is a sensor for a given analyte, base on biological active system produced in vivo or synthesized to the same structure and function. The biological system creates a unique signal corresponding to the analyte we wish to measure. In the sensor I designed, we measure the amount of hydrogen peroxide that is created by glucose oxidase when it catalyzes the reaction of glucose into gluconic acid. We do this via a catalytic reaction with Prussian blue, containing redox active copper & cyanide ions, which further catalyzes the reaction of hydrogen peroxide and changes the copper & cyanide complex structure and charge. To be precise, It is not a redox mediator, it is a catalytic biosensor. We can trace the amount of copper that changed state exactly via the current we measure over the electrodes, making the sensor pretty precise when manufactured to satisfaction.
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://steemit.com/science/@clausewitz/the-design-of-a-biosensor-for-glucose-detection
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit