In all reactions, oxidation and reduction will occur simultaneously no matter what. Under any circumstances, this will occur at the same time
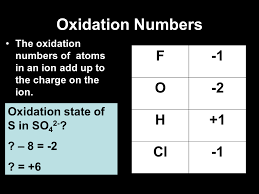
In order to keep track of electron transfers, we must use oxidation numbers. However, not all elements will have the same oxidation number in each type of reaction. In fact only 3 elements constantly have the same oxidation numbers. These elements consist of oxygen (-2), Hydrogen (+1) and Fluorine (-1). In exception, oxygen can be assigned a different number when combined in a hydroxide. For all elements that stand alone and haven't reacted, the oxidation number will always be 0. Even with a diatomic atom and not just monatomic atoms. The oxidation number will always be unless it is an ion. In that case, its oxidation number will be just as the ion reads. For example, you have a magnesium ion (Mg^2+). This automatically means that the oxidation number will be 2+.
( )
)
Now when assigning oxidation numbers to elements in a reaction, it can sometimes become difficult and challenging. But take a breath and inspect the reaction at hand. From prior knowledge you should know that all reactions have a neutral charge, unless its a polyatomic ion. So first, see if hydrogen, oxygen, or fluorine are part of the equation. If so, you then immediately know that charge, which you can then use to find the unknown numbers of the other elements. In the reactions are oxidizing agents and reducing agents. Oxidizing agents are species that cause oxidation, but are reduced. Thus, in a reaction it will lose electrons but it is the oxidizing agent but is the other way around for reducing agents. Therefore, a reducing agent is oxidized but causes oxidation
Authors get paid when people like you upvote their post.
If you enjoyed what you read here, create your account today and start earning FREE STEEM!
If you enjoyed what you read here, create your account today and start earning FREE STEEM!
Congratulations @kyleagnello! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit