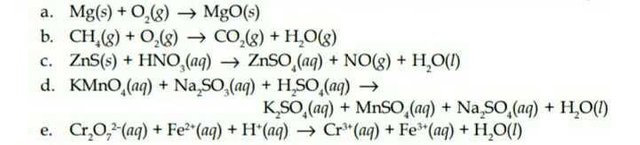You certainly know the battery, a tool that can generate electrical current.
Different types of batteries in different shapes and voltages have been numerous
made to run electronic appliances. In principle,
the current generated by the battery is caused by a chemical reaction, the reaction
redox.
In addition to batteries, the application of redox reactions is widely used inside
daily life, another example of utilization is on gilding
metal. Metal plating process, such as chromium plating on machine
motor vehicles that look shiny, using electrolysis cells.
How does the electrolysis process take place? How also the reaction that occurred
on battery?
In this chapter, you will study the equalization of redox reactions and
its application to electrochemical cells, such as the Volta / Galvani cells and cells
electrolysis and its utilization.
The concept of reduction and oxidation (redox) based on binding and
oxygen release, electron surrender and acceptance, and enhancement
and the decrease in oxidation numbers you have learned in Class room
The redox concept in the new Class room is applied in naming
so that the compound can distinguish what is the name for CuO and Cu2
O and
understand the application of the redox concept in overcoming environmental problems.
In addition, there are still many applications of oxidation reduction reactions in life
daily, such as reactions that occur in dry batteries, battery cells,
gilding and purifying of metals, as well as the prevention of corrosion.
The oxidation-reduction reaction is the ongoing reaction
electrochemical processes, ie chemical processes that generate electrical current
and chemical processes that use electric current.

How do the reactions occur? In this chapter will be discussed further
application of redox reactions in equalizing the reaction and cells
electrochemistry. In order for you to understand the application of this redox concept, do so
the following activities.
A Redox Reaction
Redox Reaction Equation