An organization in the UK is as of now leading a clinical trial of what could turn into the primary all inclusive influenza antibody on the planet - a slippery objective that would have enormous advantages for worldwide general wellbeing and wipe out our requirement for yearly infusions.
At this moment our planet is confronting its nastiest influenza season in years, exacerbated by a regular antibody that is ended up being less successful than common.
The reason we continue requiring another influenza hit each year is on the grounds that the darn infection continues transforming, with a few strains gliding around the earth at any one time.
Worldwide coordination enables disease transmission experts to settle on the few strains that show up the most pervasive year-to-year, and deliver an immunization against those.
This technique has differed levels of achievement relying upon how the infection shifts amid the immunization creation period, which can take a while.
Normally, our lives would be considerably less difficult on the off chance that we could simply get one "widespread" influenza immunization shot that could secure against most strains for a more drawn out timeframe - and now it would appear that Vaccitech, a private spinoff of Oxford University in the UK, is getting closer and nearer to that objective.
The organization, which simply reported it's scored US$27.6 million from speculators including Alphabet Inc's. investment arm GV, has just begun a two-year Phase 2 clinical trial on their general influenza immunization.
Vaccitech's licensed antibody MVA-NP+M1 has so far been effectively tried for wellbeing crosswise over 145 individuals, so the subsequent stage is to see whether it's in reality great at securing people against this season's flu virus.
Why is it so difficult to make a widespread influenza antibody? In the event that you take a gander at the regularly moving flu infection under the magnifying lens, it essentially resembles a spiky blob. Those spikes contain two sorts of protein - haemagglutinin and neuraminidase - which escort the infection through our cells, causing contamination.
It's those two quickly moving proteins that keep researchers on their toes, always contriving new immunizations that can focus on the most recent strain.
MVA-NP+M1 should work uniquely in contrast to the standard flu antibodies. It's expected to support those T-cells - a kind of resistant cell - that are as of now comfortable with seasonal influenza, and utilizations the steady center material of the infection to do as such.
Dissimilar to the proteins at first glance, in flu compose A - the most widely recognized wellspring of contamination for us - those center proteins barely change, making them a conceivably awesome antibody target.
The world-first trial is as of now testing the immunization's adequacy in 862 individuals matured 65 and more established, and the scientists are assessing to finish this exertion by October 2019.
Amid the trial, all members will get the customary influenza antibody notwithstanding either the new all inclusive one or a fake treatment shot. The specialists guess that their new immunization should expand the volunteers' assurance against this season's flu virus superior to on the off chance that they just got the yearly punch.
In the event that that turns out the case, the immunization could be pushed ahead to conclusive Phase 3 trials.
"In the event that we get positive information that shows we can influence rates of hospitalization and disease with flu at that point there is no doubt in my mind that an accomplice would take this on," Vaccitech CEO Tom Evans told Reuters.
"This could be a distinct advantage in an extremely aggressive market."
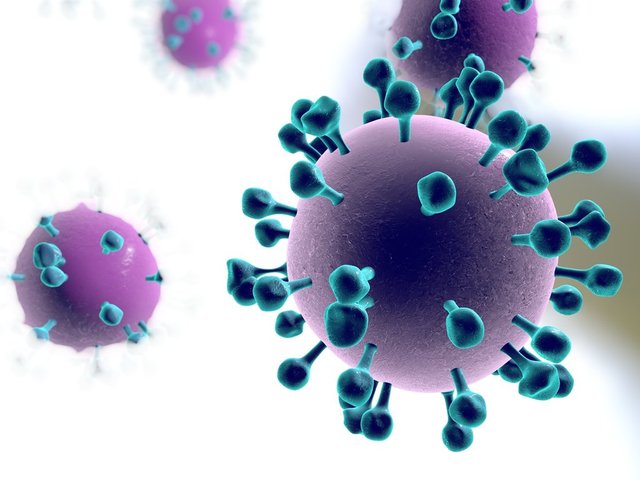
Illustration of a flu virus shows blue protein spikes on its surface (Kateryna Kon/Shutterstock)
MVA-NP+M1 isn't the main all inclusive influenza antibody underway, despite the fact that it's unquestionably the innovation that is most distant along as far as really being tried in individuals. As indicated by Evans, if all goes well, it might even be available inside 5 to 7 years.
This stuff's quite a challenge. Interesting read though. Do we have references for these info? :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit