
But what is a food texturizer?
Food additive very present in the industry, a food texturizer allows to modify the physical properties of a dish.
Usually used to modify only the texture, it can also change the flavor significantly.
This family of food additives is ubiquitous on our plate.
These texturing agents are inseparable from the prepared dishes of the industry.
You know and eat it. They are almost everywhere in reality.
There are several families: thickeners to increase viscosity; gelling agents to give a gel consistency; stabilizers to improve the stability of the food; emulsifiers to help improve the suspension of the ingredients; binders for agglomerating solid particles in powder form.

Some of these additives are in the line of sight of several scientific studies, and would be linked to several pathologies.
One of them, a widely used food additive, could seem to be involved in celiac disease.
Indeed, a bacterial enzyme used to improve the texture and shelf life of foods has been linked in several studies to celiac disease.
Myths about gluten are hard to eliminate, but celiac disease is none of that.
It is an autoimmune disease, where gluten causes the immune system to attack the intestine.
A disease that can seriously affect health, no one knows for sure what the cause is.
A study published in December 2018 in the journal Frontiers in Pediatrics indicates that a common food additive could both provoke and trigger these autoimmune attacks and calls for warning on food labels pending further testing.
MICROBIAL TRANSGLUTAMINASE AND CELIAC DISEASE
Gluten-free diets have become popular despite little or no benefit for most people.
But for 1 in 100 people with celiac disease, even a sip of bread can trigger an immune response that damages the small intestine, affecting the absorption of nutrients.
The exact cause of this autoimmune reaction to gluten - a protein found in wheat, rye and barley - is uncertain.
Specific mutations in an important immunity-related gene called HLA-DQ appear to be necessary for the development of celiac disease, as one of the two HLA-DQ variants is present in virtually all patients, but these variants are not sufficient. also present to about 30% of the general population.
As a result, a myriad of environmental factors seem to interact with the genetic risk of causing celiac disease. These cover infections, food and toxins, vaccination, drugs and surgery.
More recently, it has been suggested that food additives contribute to this.
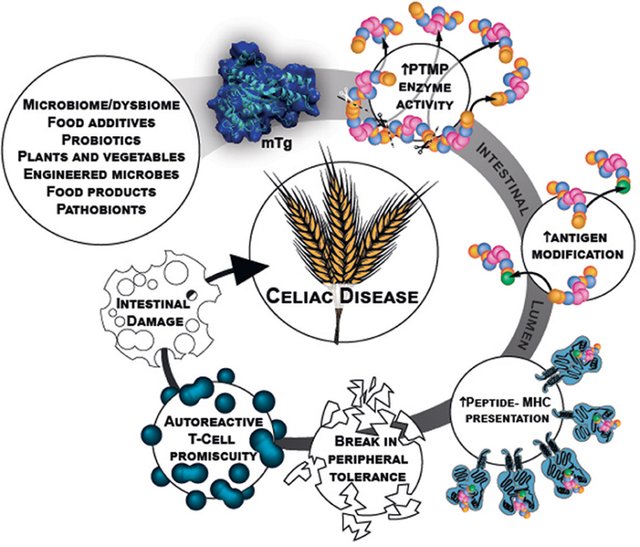
Of these, microbial transglutaminase. This bacterial enzyme, widely used in the industrial processing of meat, dairy products, bakery products and other food products, has become a culprit, according to the new study.

HOW A FOOD BINDER COULD BE OUR LOSS
Microbial transglutaminase can bond proteins together, so it is used to improve the texture, palatability and shelf life of foods.
This enzyme works like the transglutaminase produced by our body that is known to be the target of autoimmunity in celiac disease.
According to the researchers, there is a direct positive correlation between the increasing use of industrial enzymes in baked goods and the increasing incidence of celiac disease over the last four decades.
But if transglutaminase is produced normally in our tissues, and by our own intestinal microbes, what difference should it make by being a little more present in our diet?
"It's all about scale," says the study. "Our own transglutaminase has a different structure from that of the microbial type, which allows us to closely control its activity. And while relatively blind microbial transglutaminase is produced by part of our normal intestinal flora, the amount of the enzyme could be greatly increased. The microbial population is altered by factors such as infection, antibiotics or stress, or even the consumption of industrially processed foods. "
What binds gluten, transglutaminase, HLA-DQ genes, and autoimmunity?
Gluten is difficult to break down completely. This is helpful in helping baked goods to get up and stay in shape, but it is a problem for people with celiac disease.
Fragments of gluten proteins or peptides that remain after digestion are very sensitive to transglutaminase, which modifies them into a variety of new peptides.
These unusual peptides are particularly likely to resist further degradation and to be recognized as foreign by the HLA-DQ immune receptors within the gut wall, but only in those bearing the HLA-DQ variants associated with celiac disease.

In addition, gluten components relax the links between cells lining the intestine, allowing more proteins derived from gluten, as well as microbial transglutaminase, to cross this barrier and interact with immune cells.
"Microbial transglutaminase itself could also increase intestinal permeability by directly modifying the proteins that maintain the intestinal barrier," the authors of the study add.
All this raises the question: if it is protein derived from gluten that stimulates immune cells, why does the immune response target transglutaminase? And is microbial and human transglutaminase recognized interchangeably by the immune system?
The study states that "in one of our own studies we tested antibodies from the blood of celiac patients. We found that more antibodies were active against transglutaminase complexes bound to gluten fragments than against either component alone. The number of anti-complex antibodies was also the best predictor of intestinal injury in these patients. This is the case of microbial and human transglutaminase complexes, for which the number of antibodies was similar. "
In other words, microbial transglutaminase (linked to gluten fragments) could actually be the target of the immune response in celiac disease, and the attack on our own transglutaminase would simply be a case of mistaken identity.
Microbial transglutaminase present in processed foods is therefore a potential environmental cause of celiac disease.

IS MICROBIAL TRANSGLUTAMINASE A DANGER?
Being definitive about the answer seems complex for the moment.
"In the end, all we have so far are associations between microbial transglutaminase and celiac disease. To determine whether this enzyme causes or induces immune damage in celiac disease, exposure to animal models, intestinal cell lines or biopsies should be tested. ", Specify the authors.
Nevertheless, in the absence of known treatment for celiac disease, treatment depends on preventive measures, namely adherence to a gluten-free diet.
Finally, until the answer is clearer, the researchers recommend transparency and vigilance regarding the labeling of processed foods using microbial transglutaminase.
In terms of public health, products should be labeled as not suitable for people with celiac disease for more assurance.
SOURCES
- Microbial Transglutaminase Is Immunogenic and Potentially Pathogenic in Pediatric Celiac Disease. Frontiers in Pediatrics, 2018; 6 DOI: 10.3389 / fpe
Nice post. Actually this will the first time i will hear about the celiac disease
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks bro..Yeah it s a serious autoimmune disorder that can occur in genetically predisposed people where the ingestion of gluten leads to damage in the small intestine. It is estimated to affect 1 in 100 people worldwide. Two and one-half million Americans are undiagnosed and are at risk for long-term health complications.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit