I would like to talk about the subfield of geochemistry that works with radiogenic isotopes and introduce a very brief introduction of why we study them.
If you would like a quick refresher on isotopes please check out this post by @jonathanyoung or this post by @battlr, which explain them in a beautiful way.
The study of radiogenic isotopes uses isotopes that are radiogenic, or instable. That means that they decay, depending on their individual half life. A half life describes the time in which half of the existing material has decayed. In the case of uranium (U) and thorium (Th) this decay happens in decay chains, meaning the continuous, stepwise decay in different interlink elements. These interlink elements, as well as the final endmembers of the decay chains are the radiogenic elements. The common terms are parent-isotope for the element that that decays and daughter isotope for the decay product, regardless of whether they are stable or unstable and able to continue decaying.
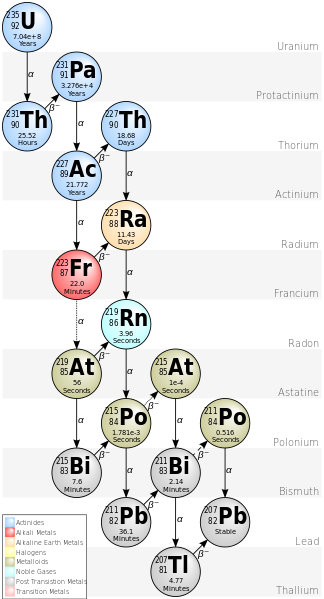
Decay chain of 235-Uranium to 2070-lead.
commons.wikimedia.org
In systems with radiogenic isotopes we use the ratio of a stable radiogenic daughter isotope against a stable non-radiogenic isotope of the same element. This is more beneficial than using the concentration for two reasons:
- They are easier to measure using a mass spectrometer.
- The concentration of an isotope does not yield much information on its own. The changes in a ratio between a radiogenic and a stable isotope allows for certain petrological conclusions.
It is important to notice that while working with radiogenic isotopes one has to consider that new radiogenic isotopes are constantly formed and the amount of daughter isotopes increases over time. This happens even in closed systems where no interaction with neighboring systems occurs.
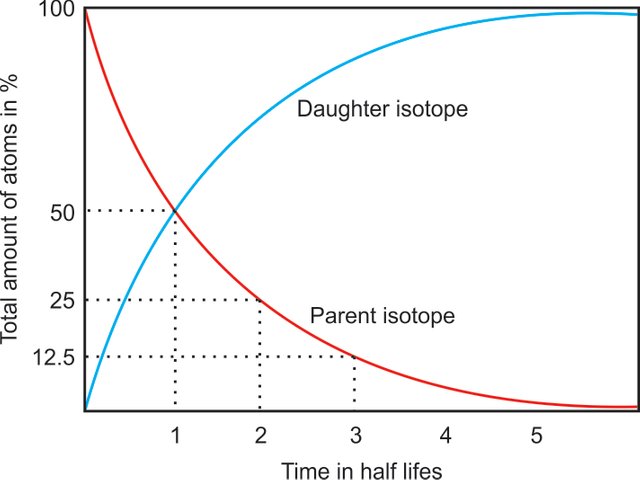
Diagram showing the change of parent and daughter isotopes with time.
This fact has advantages and disadvantages when working with radiogenic isotopes.
Due to their constantly changing behavior, radiogenic isotopes have two direct advantages:
- Because we know the half time of most radiogenic decay systems we can correlate the change in isotopic ratios with time and therefore use it for dating purposes. This process is called radiometric dating and forms the geochemical branch of geochronology.
- If two reservoirs separate, for example if a melt separates from its source rock, both reservoirs exhibit the same isotopic ratios at the time of separation. After separation the two reservoirs can contain different amounts of the parent and the daughter isotope. They then would develop differently and would display different istotopic ratios with time. It is therefore possible to reconstruct the timing and the chemical evolution of reservoirs that are separated at present day. This includes processes of magma mixing and contamination.
Because of the minimal differences in mass the ratio of radiogenic isotopes is not influenced by fractionation (separation during melting or crystallization). Changes in isotopic ratios are due to different partitioning of parent- and daughter isotopes during rock forming processes. One of the two might be preferred during melting and will be enriched in the melt after separation. The isotopic ratio itself will then change over time during continuous decay.
Sources
- Faure, G. and Mensing, T.A. (2004). Isotopes: Principles and applications (3rd edition): New York, John Wiley
- Markl, G. (2008). Minerale und Gesteine, 2. Auflage. Spektrum Akademischer Verlag
- Rollinson, H.R. (1993). Using Geochemical Data: Evaluation, Presentation, Interpretation. Longman Scientific and Technical
Diagrams
commons.wikimedia.org/wiki/File:Decay_Chain_of_Actinium.svg
You received a 80.0% upvote since you are a member of geopolis and wrote in the category of "geopolis".
To read more about us and what we do, click here.
https://steemit.com/geopolis/@geopolis/geopolis-the-community-for-global-sciences-update-4
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit