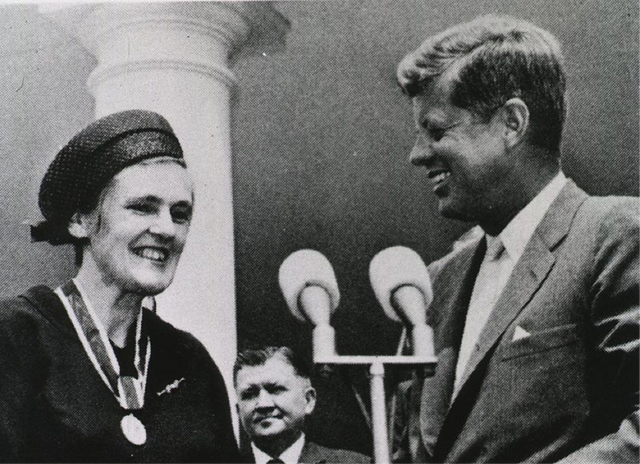
Frances Oldham Kelsey, MD, CM, a pharmacologist and doctor by trade, was the second woman in the United States to be awarded the President’s Award for Distinguished Federal Civilian Service by President John F. Kennedy in 1962 (pictured below). But why?
In 1957, a new drug hit the market, a wonder drug initially marketed as a sleeping pill that was claimed to cure anxiety, insomnia, gastritis and ‘tension’. It was later found, according to its German manufacturer Chemie Grünenthal, to have additional effects of alleviating morning sickness in pregnant women.
Based on tests conducted on laboratory animals (Greek R 2011) and later a small cohort of people (Baker R 2012), they found it was safe to use (Canada n.d.), lacked any addictive properties due to its sedative nature and no drug or alcohol interactions .
This drug – with all its benefits, and (initially at least) seemingly very few side effects – was called Thalidomide.
Thalidomide was quickly approved in more than 20 European and African countries worldwide, including Kelsey’s own country of Canada. Under ever increasing pressure from other nations and pharmaceutical giants, Kelsey, who had just begun work at the U.S. Food and Drug Administration (FDA), single-handedly refused the approval of the sale of this drug on American soil.
Her reason? A lack of evidence proving its safety. Most research into the drug at the time consisted of testimonials, and Kelsey wasn’t satisfied. (Bara Fintel 2009)
Upon seeing a physician-submitted letter in the British Medical Journal (Chanel 2016) documenting peripheral neuritis – painful tingling in one’s outer limbs – in long term users of the drug, she stood her ground, requesting more information and testing, thus effectively banning the drug’s sale beyond the volume of drugs distributed to doctors for trial use.
Kelsey’s persistence paid off, with subsequent findings that the drug led to significant fetal malformations and fetal deaths. Australian Dr William Griffith McBride CBE AO, was the first to note birth defects in parents who used Thalidomide (Milliken 1993), with many other reports soon to follow. Kelsey was hailed as a national hero in America for her work and persistence in scientific scrutiny, leading to the aversion of the Thalidomide tragedy around the country
Sadly, this was not the case around the world, with numbers ranging from 10,000 (Technology n.d.) to 100,000 babies (BBC n.d.) reported as being directly affected by the drug during what was labelled the birth defect crisis. A large community of Thalidomide survivors are still alive today. Fifty years later, it was discovered that the German manufacturer Chemie Grünenthal’s long-held claim that the effects of Thalidomide were an unforeseen tragedy, were part of a global cover-up as they had known about harmful side-effects for years prior to its ban. (Baker 2012)
Kelsey’s tireless work directly sparked reforms in drug research and approval worldwide, with reforms aimed at greater drug scrutiny, unbiased and independent controlled clinical trials, stricter drug regulation and a greater focus on evidence based practice.
Following its ban, Thalidomide was approved in 1998 following stringent procedures by the FDA, for limited use in a debilitating complication of leprosy called erythema nodosum leprosum (ENL) (Sharma NL 2007). The marketing of the drug was heavily regulated with mandatory pregnancy testing being required prior to use.

An unfortunate consequence of greater reforms, however, was the barring of women of reproductive age in general from early stage trials (FDA 2016), a knee-jerk reaction to ensure a catastrophe of this magnitude would never be repeated, which was finally repealed in 1993 (FDA 2016) and later reformed in January 2016 (NIH 2016) by the FDA and National Institute of Health in the United States after overwhelming evidence regarding females experiencing significant side effects to drugs that were considered safe for use in male study populations. Work on reducing medical research gender gaps continues today (Women’s Health Victoria 2016).
There is no doubt that Kelsey’s unwavering persistence, vigilance and eye for medical scrutiny had a direct impact in the evolution of medical research and had positive impacts in the trend away from eminence-based practice to the evidenced-based practice we see today.
Hailed as a hero in the advancement of science and medicine, Kelsey passed away after receiving an Order of Canada award in 2015.
This article was originally posted by myself on the Doctus Project by myself. This line is to certify that the original author of the article is reposting on Steemit for the Steemit community. Original article can be found here: http://www.doctusproject.com/2017/03/12/frances-oldham-kelsey-md-cm-an-unwavering-commitment-to-evidence-based-medicine/References
- Baker R, McKenzie N. 2012. Australian women used as human guinea pigs. July 27. http://www.smh.com.au/national/australian-women-used-as-human-guinea-pigs-20120726-22voq.html.
- Baker, Nick McKenzie and Richard. 2012. The 50-year global cover-up. July 26. http://www.smh.com.au/national/the-50year-global-coverup-20120725-22r5c.html.
- Bara Fintel, Athena T. Samaras, Edson Carias. 2009. THE THALIDOMIDE TRAGEDY: LESSONS FOR DRUG SAFETY AND REGULATION. July 28. https://helix.northwestern.edu/article/thalidomide-tragedy-lessons-drug-safety-and-regulation.
- BBC. n.d. Effects of thalidomide ‘were horrific’. http://www.bbc.com/news/health-27420736.
- Canada, Thalidomide Victims Association of. n.d. Thalidomide The Canadian Tragedy.http://www.thalidomide.ca/the-canadian-tragedy/.
- Chanel, Documentary. 2016. Thalidomide Story. Accessed 2017. http://thalidomidestory.com/story/timeline/timeline/+/kelsey-reads-florences-letter-in-the-british-medical-journal/.
- FDA. 2016. Gender Studies in Product Development: Historical Overview.https://www.fda.gov/ScienceResearch/SpecialTopics/WomensHealthResearch/ucm134466.htm.
- Greek R, Shanks N, Rice MJ. 2011. “The History and Implications of Testing Thalidomide on Animals.” The Journal of Philosophy, Science & Law.
- Milliken, Robert. 1993. ‘Thalidomide doctor’ guilty of medical fraud: William McBride, who exposed the danger of one anti-nausea drug, has been disgraced by experiments with another, writes Robert Milliken in Sydney. February 20. http://www.independent.co.uk/news/world/thalidomide-doctor-guilty-of-medical-fraud-william-mcbride-who-exposed-the-danger-of-one-anti-nausea-1474190.html.
- NIH. 2016. Sex as a Biological Variable: A Step Toward Stronger Science, Better Health.https://orwh.od.nih.gov/about/director/messages/sex-biological-variable/.
- Sharma NL, Sharma VC, Mahajan VK, Shanker V, Ranjan N, Gupta M. 2007. “Thalidomide: an experience in therapeutic outcome and adverse reactions.” The journal of dermatological treatment 335-40.
- Technology, Emory Libraries & Information. n.d. All Americans Will Pull Together…The Federal Government’s Evolving Role in Dealing with Disaster: Thalidomide . http://guides.main.library.emory.edu/c.php?g=50422&p=325039.
- Women’s Health Victoria. June 2016. http://whv.org.au/publications-resources/clearinghouse-connectors/chc-women-in-clinical-trials.
Congratulations @sperera! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPDownvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @sperera! You received a personal award!
Click here to view your Board of Honor
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @sperera! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit