Solar energy has the potential to provide a long-term, clean, and sustainable solution to the world's energy dilemma by meeting the growing need for energy.
The energy emitted by the Sun reaches the Earth in an hour, which is enough to meet the entire world's energy requirements for a year. Solar energy has the potential to provide a long-term, clean, and sustainable solution to the world's energy dilemma by meeting the growing need for energy. However, difficulties like as the variability of solar energy during the day and the inability of solar energy to be stored must be solved first and foremost.
In fact, it is possible to identify a naturally occurring example of solar energy storage that is both efficient and inexpensive. It is through photosynthesis, or the conversion of solar energy into chemical compounds that plants can use to meet their energy requirements, that plants are able to store solar energy in the form of chemical bonds.
Fuel cells, which convert chemical energy into electrical energy through the reaction of hydrogen and oxygen, generate energy through the reaction of hydrogen and oxygen. The hydrogen required for fuel cells, on the other hand, is often produced by utilizing electrical energy to decompose water, which is a process known as electrolysis.
In recent years, scientists have been investigating the possibility of using solar energy for this purpose, with promising results. However, in order for these technologies to be helpful, the methods that are developed must be extremely efficient, and the materials that are used must be easily available, inexpensive, and long-lasting.
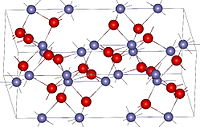
Image Crystal structure of hematite
Using the world's most abundant elements, iron and silicon, scientists have developed a new technology that produces hydrogen by decomposing water under the influence of sunlight, effectively storing solar energy. The technology is being developed by researchers at the University of California, Berkeley. It was Hematite that was used as the electrode material by the researchers.
Hematite is an iron oxide complex on the surface of which water is oxidized, or given off electrons, resulting in the production of oxygen gas. To create the electrode, silicon was employed, which produces hydrogen gas by reducing water or obtaining electrons, as well as by reducing water. The efficiency of the new procedure is not quite at the level that it should be, but the results indicate that progress has been made in comparison to previous investigations.
Reference:

Hello @tvm, your post has been supported by @jimah1k using @steemcurator07 curation account, which we are using for #science, #technology, and #computing theme tags.
Thank you for making a post in the #science category. We appreciate the work you have put in this post.
We have analyzed your post and come up with the following conclusion:
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thank you @jimah1k
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit