Two-dimensional materials are one of the most active study topics nowadays. These materials have a structure that is comparable to that of crystalline solids. They are, however, two-dimensional rather than three-dimensional, as are conventional crystalline solids.
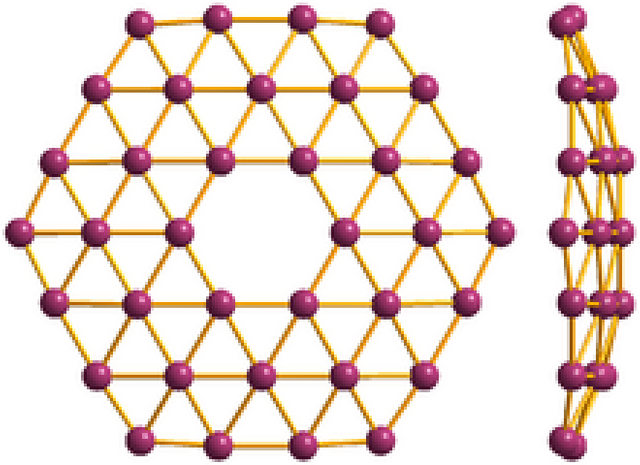
ImageStructure of Single Layer Material
We live in a three-dimensional world. This space is occupied by solids, liquids, and gases created by the combination of atoms. Two-dimensional, or single-layer, materials are one of the most active research areas today. These materials have a structure that is comparable to that of crystalline solids. They are, however, two-dimensional rather than three-dimensional, as are conventional crystalline solids.
Atoms in crystalline materials form a three-dimensional, organized structure that fills a spatial volume. The material is multi-layered, with strong and weak connections between the layers. Diamond and graphite, for example, are three-dimensional crystalline solids created by the interaction of carbon atoms. In graphite, which has an extremely flexible structure, the carbon atoms are bound to each other in layers to form a hexagon. The weak bonds between the multiple layers are the explanation for the material's softness.
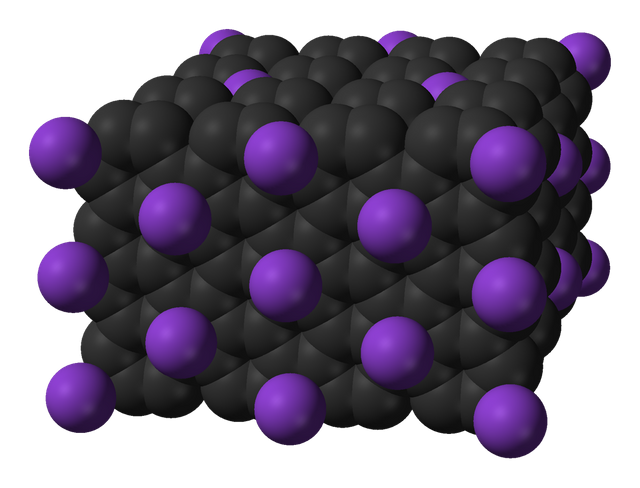
ImageStructure of graphite
Carbon atoms in a layer establish strong bonds with their four closest neighbors in layers above and below it in diamond, one of nature's hardest minerals.

ImageStructure of the diamond
Two-dimensional materials, like three-dimensional materials, have a regular structure. The atoms, on the other hand, are contained within a single layer in these materials. There are no layers that are connected by strong or weak links. Consider graphene, which is made up entirely of carbon atoms.
Theoretical approaches have assessed the stability of roughly 700 single-layer materials to yet. Some of these materials were also created artificially. Graphene was the first single-layer material to be created in a lab setting. Andre Geim and Konstantin Novoselov used sticky tape to break off a layer of graphite, which they then put onto a silicon plate in 2004.
For their revolutionary work on graphene, Geim and Novoselov were awarded the Nobel Prize in Physics in 2010. Other monolayer materials made in the lab include borophene, which is made up of boron atoms, germanene, which is made up of germanium atoms, and bismutene, which is made up of bismuth atoms. Two-dimensional compounds are also present. For instance, consider (CH) n, where n is a big number. Graphane is a carbon and hydrogen atom-based molecule with the chemical formula graphane.
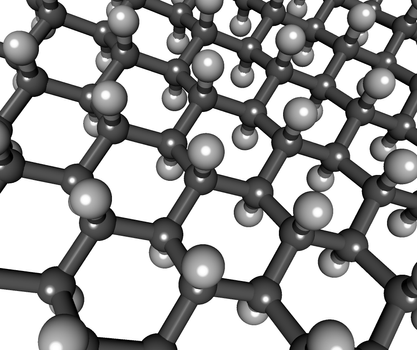
ImageStructure of graphene
Two-dimensional materials are rarely employed on a wide basis in today's world. Many two-dimensional materials, on the other hand, are being studied, and they are regarded to offer a lot of potential in industry and technology. Graphene, for example, is hundreds of times stronger than many steels of the same weight. Graphene is also the material with the highest thermal and electrical conductivity. It can carry a million times the current density of copper.
Stannene is a monolayer substance made up of tin atoms that has been theoretically predicted to be stable but has yet to be manufactured. Stane is an example of a material characterized as a topological insulator (with insulating bodies and conductive edges). Furthermore, according to estimates, stanen has the ability to collect and degrade air pollution-causing chemicals such as NOx and COx.
Many other single-layer materials, in addition to graphene and stannene, are expected to be beneficial in a variety of technologies, ranging from semiconductor devices to solar cells and water purifiers. The difficulty of utilizing two-dimensional materials with three-dimensional materials is one of the most significant constraints restricting their utilization today.
References: