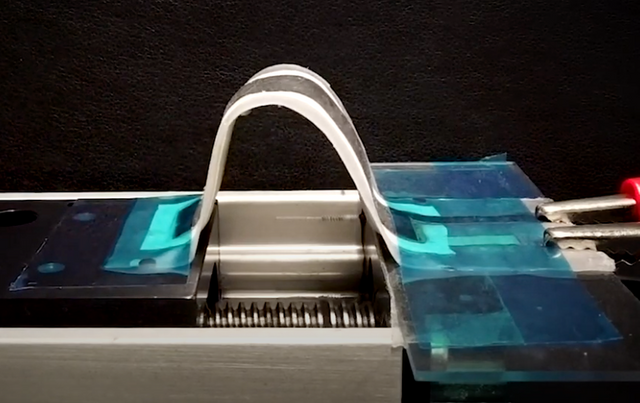
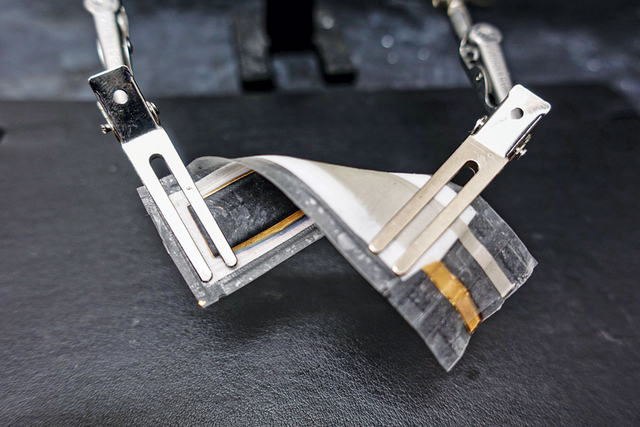
As industries such as mobile phones and electric cars develop, the demand for lithium batteries is gradually increasing. After so many years of study and development, the evolution of lithium batteries is difficult to predict. You had to charge your phones every day till today. The cruising range of a car is frequently a source of concern. As a result, engineers are working on the next generation of battery technology. Today we'll look at a rechargeable silver-zinc oxide battery. It offers a 5 to 10 times better energy density and is much more adaptable than a lithium battery.
However, the principal application scope of this new battery is not in mobile phones or electric cars; rather, it is largely used as a button battery replacement. Button batteries are, for the most part, disposable. These batteries are both small in size and capacity. They're mostly used to power some of the most crucial electrical devices. Because of their small capacity, the current drawn by these button batteries is frequently low.
However, as the 5G and Internet of Things industries increase, so does the demand for high-current button batteries. As a result, some button batteries are suitable for these electrical devices that cause drowsiness. Although they are per square cm lithium batteries, many of them are made of lithium batteries. The rechargeable silver-zinc oxide battery has a 50 mA per square cm energy density, but the lithium-ion battery only has a 5 mA energy density.
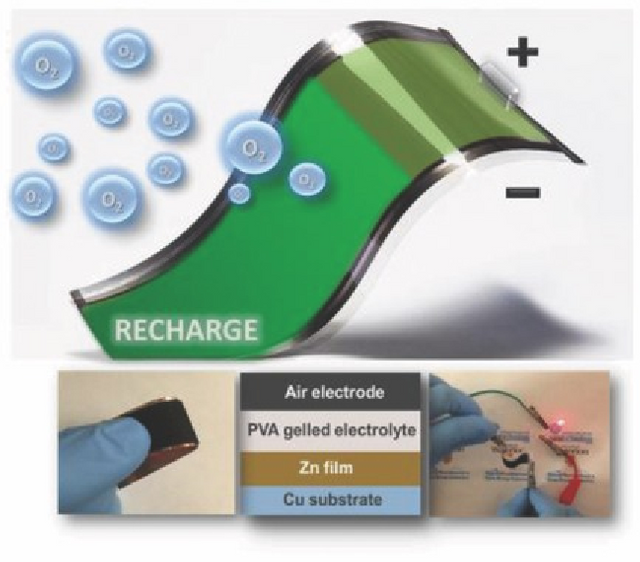
Rechargeable silver-zinc oxide batteries have a higher capacity than typical lithium-ion button batteries for the following reasons:
One explanation is that its impedance, or the circuit or device's resistance to alternating current, is still much lower. The lower the impedance, the better the battery's high-current discharge capability.
The second concern is the chemical properties of the silver oxide-zinc (AgO-Zn) utilised. The bulk of commercial flexible batteries use Ag2O-Zn chemistry. As a result, their cycle life is typically limited, and their capacity is limited. AgO has a bad rep for being unreliable. The AgO cathode material in the new battery, on the other hand, has a proprietary lead oxide coating that improves AgO's electrochemical stability and conductivity, and the chemical composition of AgO-Zn contributes to the battery's low impedance.
AgO has never been used in screen-printed batteries before due to its high oxidising capabilities and quick chemical breakdown. Experimenting with several solvents and adhesives, the researchers discovered an ink formulation that allowed AgO to be produced. As a result, a new battery has been developed.
The rechargeable silver-zinc oxide battery material is heat-sealed and printed on a chemically stable, flexible polymer sheet with a high melting point (about 200 degrees Celsius). The flexible battery also includes a stacked screen-printed layer that contains the current collector, zinc anode, AgO cathode, and their corresponding separators.
The rechargeable silver-zinc oxide battery isn't perfect yet. It is still developing its next-generation products. It has a lower price, lower impedance, faster charging speed, and other attributes that make it appropriate for 5G or soft robot equipment that requires high power, adaptability, and flexibility.