 Source |
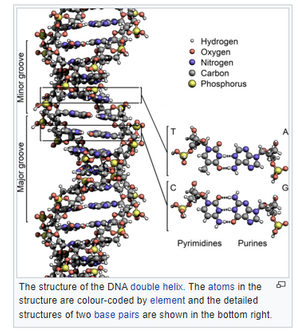
This is a representation |
DNA is like a set of blueprints that contains all the information necessary to build an organism. There are several types of RNA but essentially they are one helix in shape and some will carry the information from a section of the DNA to creates other particles at sites like the ribosomes. Viruses hijack a cell's normal processes and transfer their own blueprints to a cell's "manufacturing plant" (eg ribosomes) to create new viruses.
The drug Molnupiravir is the derivative drug N4-hydroxycytidine. Its antiviral action is by corrupting the viral RNA replication process. It damages the RNA of the virus to cause mutations.
In April 2020, Rick Bright, who was removed as head of the Biomedical Advanced Research and Development Authority (BARDA) before the approval of the drug, submitted a whistleblower complaint asserting that Ridgeback pressured BARDA to provide funding to manufacture EIDD-2801 despite Bright's concerns that similar drugs in its class have mutagenic properties. A previous company, Pharmasset, that had investigated the drug's active ingredient had abandoned it over similar concerns.
In June 2021, the US Department of Health and Human Services committed to buy US$1.2 billion worth of molnupiravir from Merck if it receives FDA approval.
On 1 October 2021, Merck stated that an independent advisory board that has been monitoring the COVID-19 clinical trial recommended that the study be stopped early because of convincing evidence of the drug's benefit to patients, reducing the risk of hospitalization or death by around 50%.
At this stage, Molnupiravir has not been fully tested but has been fast-forwarded through the process. However, compare this compound that has a much higher level as prophylaxis.