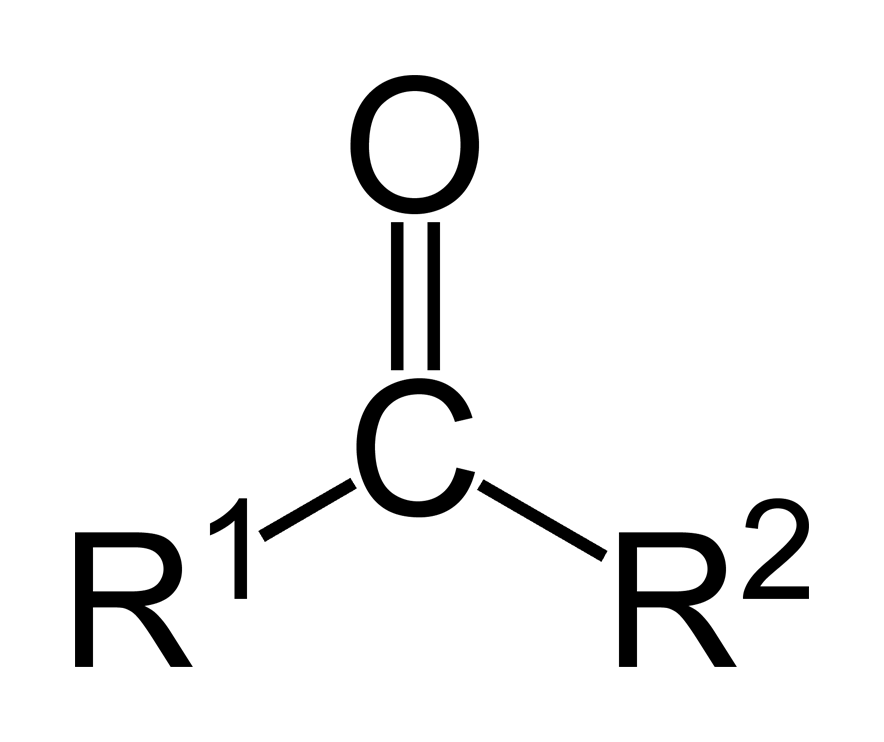
A ketone
defined as two R-groups (traditionally hydrocarbon chains) with a carbon double bonded to an oxygen.
I first remembered the term "ketone" by the functional groups' resemblance to a bike key
Synthesized from an alcohol (R-OH-R) through oxidation by PCC, DMP, KMnO4 (with acid), or H2CrO4. Can also be synthesized through ozonolysis (1. O3, CH3Cl3 2. Zn, OHAC) of an alkene (two carbons with a double bond). Alkynes (two carbons connected through a triple bond) can by converted through mercuration (HgSO4, H2SO4, H2O) or hydroberation (1. BH3, THF 2. O2H2, OH-) if the alkyne is terminal.
They are very reactive in acidic condition, because of the inductive effect of the oxygen, which withdraws the electrons from the carbon with which its attached, giving that carbon (referred to as the carbonyl carbon) a partial positive charge, and the oxygen a partial negative. The protons (H+) from the acid further activate the carbonyl carbon, making it the perfect target for a nucleophilic attack. Without acidic conditions, the carbonyl carbon still holds enough of a partial charge, that if a nucleophile is strong enough, a nucleophilic addition reaction may occur.
Aldehydes are similar (Aldehyde is also defined by it's C=O, but with one of the R groups being limited to just a hydrogen). They can by synthesized from a primary alcohol using a weaker oxidizer (strong oxidizer would result in carboxylic acid), or through the reduction of an ester by DIBAH.
This particular functional group is of no curiosity to me and I imagine it's not to you either. Organic chemistry is an intense class, though interesting and useful field of study if one is trying to synthetically create organic molecules and is willing to understand the mechanisms by which these reactions occur. The course itself requires no attention to the meaning or depth of these reactions and it is only required we memorize them. I imagine anyone who decides to pursue organic chemistry finds these reactions interesting and important, and i applaud them. As for myself, I'm just trying to get an A and stuff the reactions into my brain as efficiently as possible so I can go back to practicing my beloved calculous--a logical and reliable subject that required minimal memorization and is more focused on understanding. But alas, my organic chemistry test is on Friday and so organic chemistry I will memorize.