hi everybody, today we will talk about metals
but first: what is a metal!!!?

a metal is a simple substance that possesses certain characteristic properties that can be explained by the following fact:
the atom of a metal tends to lose one or more electrons to give a positively charged cation, so a metal is an electropositive element.
The Greeks and Romans knew only seven metals, gold, silver, iron, copper, lead, tin, and mercury.

Today chemistry, which counts 49 metals, can only define them in a very general manner, saying that they are simple bodies, liquid or solid, attackable by oxygen or by acids.
.jpg)
1-The Structure of Metals:
Metals do not usually have a homogeneous structure even when they are very pure; a microscopic examination shows that they are formed by an assembly of small crystals (micro crystals)
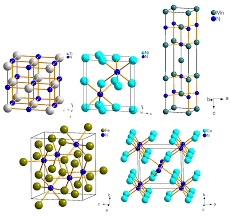
An X-ray study shows that each crystal is formed by a regular and ordered arrangement of identical atoms. each atom can release one or more electrons (this is the essential property of metals).
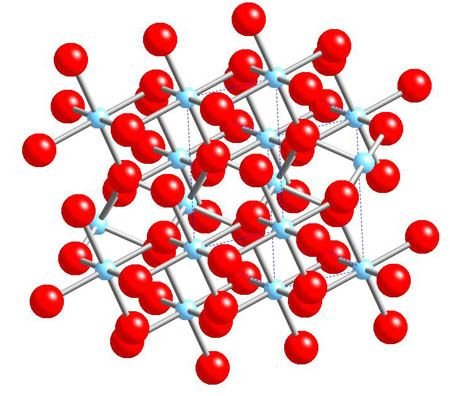
2-Oxidation of metals:

it is the essential reaction since it results from their definition.
All the metals we will study are attacked by oxygen.
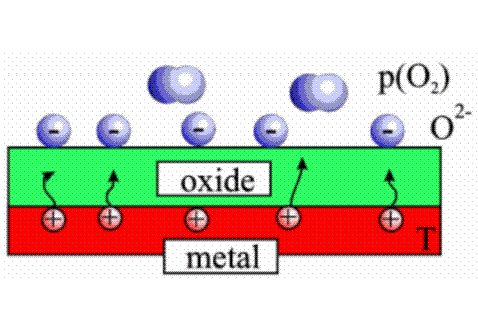
in pure oxygen, in particular, most of them can burn when they are red.
We have observed many examples: combustions of sodium, iron, magnesium .....
The color of the flame varies according to the nature of the metal.



b-Oxidation by chlorine:
All metals are attacked by chlorine at a higher or lower temperature, when this oxidant is in excess, the metal gives up the maximum of electrons and is transformed into the most positive ion.
c- Oxidation by sulfur:
the metals are attacked by sulfur and transformed into sulphides, it is also in the form of sulphide that many of them exist in the natural state (iron sulphide, sulphide of zinc, sulphide of lead, sulphuret of mercury....)
METALS ARE ELECTROPOSITIVE ELEMENTS, THEY ARE EASILY OXIDIZED
3-Physical properties of metals:
a) Color:
all the metals have a great power reflector of the light. when the surface is not oxidized; 'metallic flake' is characteristic; the color can be varied (red for copper, yellow for gold, gray-white for silver, blue-gray for zinc .....)
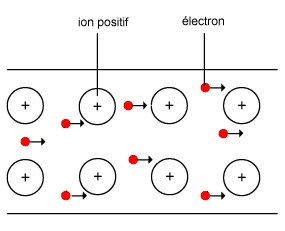
b) Thermal and electrical conductivity:
Metals are good conductors of heat and severe electricity to the free electrons of their crystal lattice.
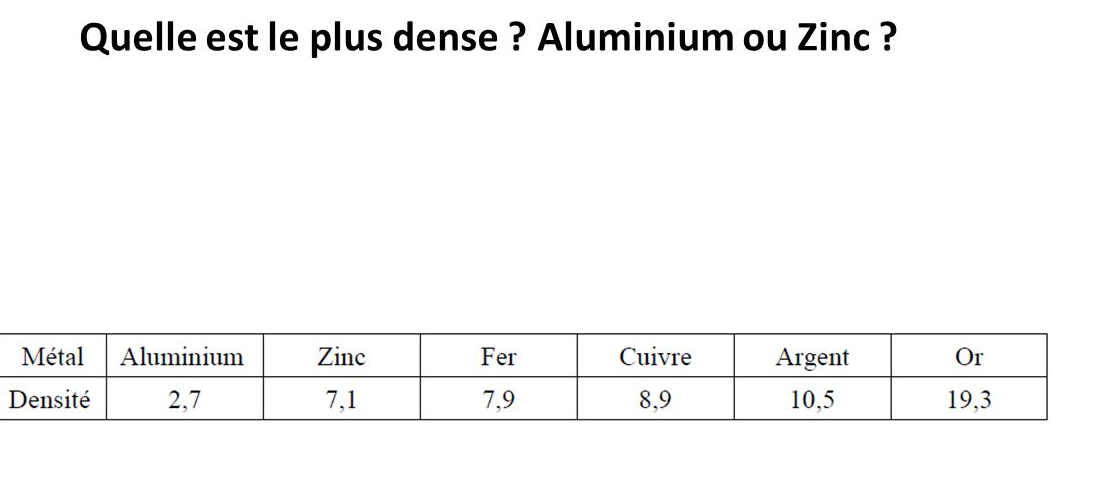
c) Density:
Light metals such as aluminum and magnesium have a density of less than 3; exceptionally, they may be less dense than water (sodium or potassium)
Heavy metals have a density greater than 10 or close to 10 as platinum, lead, mercury ....
4-Alloys of metals:


Base metal production
Metals are commodities without which a modern industrialized economy can not exist. In particular, iron and steel are ubiquitous and indispensable for dealing with basic needs such as housing and transport. Base metal production involves the smelting and refining of ferrous, precious and non-ferrous metals from ores or scrap using metallurgical techniques. It also includes the production of metal alloys and superalloys obtained by adding certain chemicals to pure metals. The product of smelting and refining is usually used in the form of ingots to manufacture, by rolling, drawing and extruding, foils, ribbons, bars, rods, wires, tubes and metal pipes as well as hollow sections, or in liquid form for producing molds and other basic metal products.
Steel and Non-Ferrous Metals Industry:

In the steel and non-ferrous metals sectors, bearings find their place in a wide variety of plants and equipment used in the upstream and downstream processes: from the material park to the manufacturing facilities, rolling and refining steel.
These bearings operate in a steel mill's own work environment and are particularly exposed to particulate contamination, as well as water and heat. The bearings must also withstand high loads or shock loads, vibration and extreme operational speeds combined with rapid acceleration and deceleration. All these demanding operational conditions that are not met with in other applications.
In all, there are only six metals used very frequently:
Iron, atomic symbol Fe.
Copper, atomic symbol Cu.
Zinc, atomic symbol Zn.
Aluminum, atomic symbol Al.
Gold, atomic symbol Au.
Silver, atomic symbol Ag.
thank you for your attention
have a nice day.
Congratulations @benainouna! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPDownvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thanks for information.. upvoted..
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
مواضيع رائعه وشيقه ... ان شاء الله سوف يكون لك اسم على هذا الموقع
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
شكرا لك أخي, اتمنى لك النجاح
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
تسلم موضوع جميل
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
الله يخليك أخي
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit