
Many people are talking about calories all the time, but, on many occasions, they do not know what a calorie really means. Some have even said that a calorie is a physical object. This is a serious error. Specifically we might say that: A calorie is the amount of energy needed to heat a gram of water to a degree Celsius.
So let's say you have an apparatus to do this kind of experiment. Put what you are going to burn in it, burn it and measure the amount of heat emitted. Or rather measure how many grams of water can heat 1 degree Celsius. Or how many degrees C can raise a gram of water, I suppose. So let's say we burn this and lift 5 grams of water in 1 degree Celsius. That's like 5 calories. If you raise 10 grams of water in 1 degree, that's 10 calories. If you raise 1 gram of water by 10 degrees, that's 10 calories.
Calories vs. Kilocalories: Clarifying the terms
Common strains tend to use the terms calories (cal) and kilocalories (kcal) incorrectly.
The kilo prefix means 1000 in metric units. Therefore 1 kilocalorie equals 1000 calories. This works equally when we say 1 kilogram equals 1000 grams.
The people use these terms without really knowing their meaning; then use these terms as synonyms. Scientists do not usually make this mistake, however, the notation used by them is a bit more complex because naming and writing convention 1 calorie (with a lowercase 'c') actually represents 1 calorie and 1 calorie (with a capital 'C ') represents 1 kilocalorie. For example:
1 calories (Big C) = 1 Kilocalorie (kcal) = 1000 calories (little c).
People do not pay attention to many medical terms they use daily.
For example, someone could write that they were eating 2000 calories (uppercase C) and this is the same as eating 2000 calories, but it is not the same as eating 2000 calories (which is only 2 calories or 2 calories). Can you notice the error? Then it is very important to know what the calories are and how they are represented by the nomenclature of standard use in the food industry. This way you will have a better way of understanding all the nutritional information that is printed on the packaging of the food products that you consume each day.
Calories vs. Kilocalories: Part 2<h/3>

As we said in the previous section this is really important because, when we talk about a diet, we are always talking about kilocalories or calories as if they were the same thing.
Another simple example we could give you would be: an apple. An average apple contains 100 kcal (100 calories with a large "C"), which means it contains 100,000 calories (little "c"). So, according to mathematics, a 2000 calorie / calorie diet would technically contain 2,000,000 calories (little "c").
Some journals, specializing in dietary products and diets, know that most people do not know how to make conversions between energy units, so they publish diets and other recipes with units such as kcal / kilojoules or kcal / megajoules, which may confuse most the people who read these tips and recipes.
The pump calorimeter
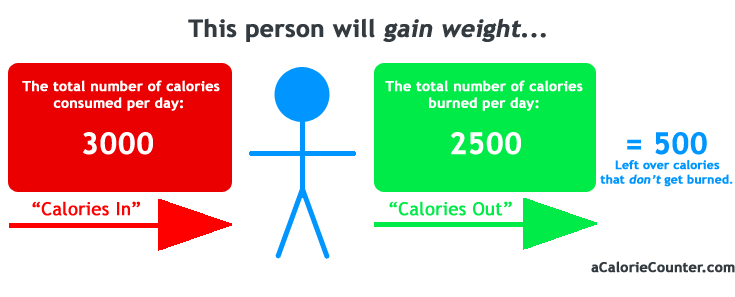
We all know that when it comes to consuming food or dieting, we also talk about calorie and energy consumption. Nutrition speculators have often explained that an average person might need to consume 2000 calories or a little more per day to maintain their weight or that all those people who wish to lose body weight should eat 500 calories below the daily energy expenditure to lose weight and that people who need to gain weight should consume 500 calories a day in order to gain weight and this is the fundamental basis of the energy balance equation. But what does this mean?
In the laboratory, you can put nutrients or foods in what is called a calorimeter pump; in this way it is possible to measure the amount of heat emitted by the food. This is the information required to know the caloric value of that nutrient or food. Again, the calorie in this regard is only a defined measure of heat.
Decades ago, a researcher called Atwater did this research aimed at knowing the calorific value for individual nutrients and derived what are called the Atwater factors. Traditionally they are given at 4 kcal per gram of protein and carbohydrate, 9 kcal per gram of dietary fat and 7 kcal per gram of alcohol. However, not all proteins, carbohydrates or fats produce exactly that amount of heat when it burns, but they are all in that range on average.
Many experts agree, therefore, that the caloric values of food are merely a representation of the thermal energy that is released when they are burned. If burning a food raises X grams of water by AND degrees Celsius, then CALCULATE the calorie level.
Denying Pump Calorimetry
Thousands of scientific studies over time have shown that foods vary in the way they are absorbed efficiently by the human body and this could vary somewhat for any food. Fats are very efficient to be digested because they have approximately 97% digestion efficiency, proteins could vary approximately 85-95%, while animal proteins that show higher values than vegetable proteins and carbohydrates can be so low as 80% due to fiber intake. Higher carbohydrate diets produce more fecal material than low carbohydrate diets.
When metabolism occurs in the human body, some of the energy provided by food may be lost. This effect is called Food Thermic Effect (TEF), Specific Dynamic Action (SDA) or Diet Induced Thermogenesis (DIT). These are all slightly different things, but, similarly, you should think of these terms to define the thermal value of food that has been lost during metabolism before the calories are "stored" in the body.
The nutrients are used in different ways by the human body. For example, the fat we consume in our daily feed is stored with a very high efficiency, almost 100%, after digestion, it also has a small TEF value, while the carbohydrate in the diet will lose about 23% of its energy if they become fat.
And what the CICO deniers will point out is that because of these factors the Atwater values do not matter or do not matter. The 4 cal / gram of protein will not be 4 cal / gram in the body due to digestion / absorption, TEF, etc.
In a calorimetric pump, the protein has a caloric value that is actually greater than 4 cal / gram. The value of 4 calories per gram has already been adjusted for the fact that the body will handle it somewhat differently than if you burn it in the laboratory. Therefore, as is the case mostly for this particular group of nimrod, it is a non-argument based on a non-understanding of what numbers actually represent.
Something to remember about calories
Remember some very important things, the first is the fact that calories are not a real physical object, but simply a defined value to measure certain things in our body. If you take a bike ride, it is possible to define and measure the watts produced by your legs when performing the act of muscle contraction to be able to turn the bicycle wheels. In addition, muscle contraction is fueled by ATP (adenosine triphosphate) and that ATP is produced by the metabolism of stored carbohydrates of fat within the muscle. Watts are then an external measure, which is capable of representing the metabolism of a living being. It is simply a power for the fuel used by the body to generate muscle strength.
In fact, you are able to get a great calorimeter pump you could study a person, exercising or not, in what amounts to a large calorimeter pump and measuring your calorie expenditure in the same way you would measure it by burning something: by measuring the amount of heat they emit and determining how much the water temperature would increase based on the definition of a calorie.
You've heard many times about horsepower, you've heard about new vehicles and motorcycles that have powerful engines and boast of having hundreds of horsepower. This is defined, curiously, as the amount of energy produced by a single horse. So you could say that a car produces 50 horsepower, the same amount of energy that 50 horsepower together would produce. It is a defined amount that ultimately represents how the engine is working based on the combustion of gasoline.
A Natural Proxy for Nutrients and Foods
Food does not contain a certain number of calories. Also, it is not correct to think that a person is eating a daily 2,000 calorie diet because they are not eating calories in the sense of those calories that are a physical thing.
This is only semantic errors, because it is not possible to eat calories.
Obviously, this is just a phrase not to explain more complicated tasks, because we eat animals, which contains nutrients that are processed by the body to generate the energy we need, which can be measured in calories.
Calories are simply a power for food / nutrients in the diet. Nothing more and nothing less. No, they do not exist in the sense of being a real physical object. Rather, they are real in the sense that they represent the end result of the metabolism of something real.
And it would be tedious to continually say: "An apple contains nutrients which, after digestion and TEF, represent heat energy during oxidation to raise a certain number of grams of water by a certain number of degrees C which can be calcaulted as 100 calories ". Instead we say "An apple contains / has 100 calories." I wish their emails were so concise, but I still do not read them any more.