Specialists from the Massachusetts Institute of Technology and the University of California at San Diego clarified the mechanism of cell destruction in aneuploidy.
Normally, the human genome consists of 23 pairs of chromosomes. In the course of mitosis, these structures bifurcate and divide equally among the daughter cells. Failure at this stage leads to aneuploidy - a change in the karyotype, in which the number of chromosomes is more than once haploid (with a single copy of the chromosome) set. Such a violation can lead to spontaneous abortion and hereditary syndromes, for example Down's syndrome, which is characterized by the presence of an extra copy of the 21st chromosome. In addition, genetic defects are inherent in many malignant, especially solid, tumors. So, often the variations in the number of copies of genes are associated with the growth of their resistance and metastasis.
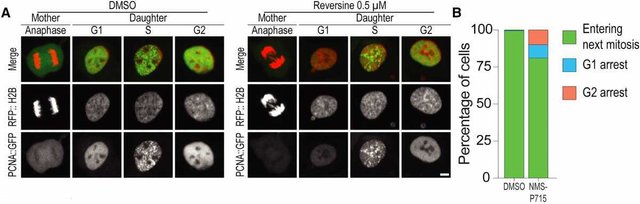
Nevertheless, such genetic instability is relatively rare. It is known that in violation of segregation of chromosomes, the proliferation of defective cells is suspended and the process of apoptosis is initiated. The exact mechanism of the immune response in this case has so far remained unclear. To fill the gap, American biologists conducted a series of experiments. At the first stage, they grew "immortal" retinal pigment epithelium cells hTERT (can serve as a cancer model) in the presence of toxins and observed anomalies of chromosome divergence during fission. The analysis showed that 80 percent of such cells continued to proliferate, despite the appearance of defects.
The discrepancy between chromosomes in the presence of a neutral substance (on the left) and aneuploidy in the presence of a toxin (right). Diagram B shows the proportions of cells that have continued (green) and ceased (blue and pink) division / © Stefano Santaguida et al., Developmental Cell, 2017
As a result, model cells formed complex karyotypes with aneuploidy in the absence of apoptosis. Meanwhile, about nine percent of the cells stopped the division and proceeded to develop cytokines-information molecules mediating intercellular signaling, including in apoptosis. Scientists have suggested that the production of these compounds can provoke the activity of natural killers. They are large granular lymphocytes that are cytotoxic to cancerous and infected cells. The hypothesis was confirmed: the damaged cells exhibited an increased number of specific biomarkers (MICA and MICB) for binding to lymphocytes.
Then the researchers mixed natural killers with defective cells. According to the results, after 6-12 hours lymphocytes began to destroy aneuploid cells, without affecting the cells with a full set of chromosomes. According to the authors, the data obtained clarify the mechanism of recognition and utilization of cancer cells at an early stage. It is noteworthy that the damaged biomaterial itself signaled mutations (via the NKG2D receptor on the lymphocyte membrane), but most of these cells did not bind to the killers. An additional experiment to inhibit the NKG2D receptor confirmed that such antigens prevent apoptosis.
Details of the study are presented in the journal Developmental Cell.