INTRODUCTION TO USERS:
Congratulations on completing your first worksheet! How did you find it? I bet it was a bit challenging because it's your first time accomplishing a lesson independently. But now, I believe that you already have an idea on how to accomplish this one. Your lesson last week was all about the uniqueness of our mother Earth. In this lesson, let us start appreciating one of our planet Earth's common and amazing features, "Rocks and Minerals.This worksheet is all about rocks and minerals. It tackles about the physical and chemical characteristics of a rock-forming mineral, the three classifications of rocks, and how weathering products are transferred through deposition and erosion.
Learning Area: Earth and Life Science 11
Quarter: One
Most Essential Learning Competency:
- identify common rock-forming minerals using their physical and chemical properties (S11/12ES-Ia-9);
INTRODUCTION TO THE LESSON:
Think of things or products that contain a mineral/s. Minerals are the building blocks of rocks. You could find more than two minerals in a rock. However, since rocks are exposed to other substances, it is composed of a combination of minerals and organic substances.
How are you going to identify a mineral? How will you differentiate a mineral from other substances that is not a mineral? Here are the criteria that you could use to identify one.
FIVE IDENTIFICATIONS OF A MINERAL
1. It is a substance.

- A mineral should have a specific composition and properties.
- All mixtures are not minerals, while some elements (mainly native elements) and compounds are minerals.
- Native elements are pure, naturally existing elements that are identified as minerals. There are 19 known native elements. These are gold, osmium, platinum, iridium, tin, iron, copper, zinc, lead, mercury, chromium, silver, arsenic, bismuth, selenium, antimony, tellurium, arsenic, carbon, and sulfur.
2. It is a naturally occurring, solid substance
- All human-made substances are not minerals.
- Also, all liquid substances and gases are not
under the category of a mineral
3. It is inorganic substance
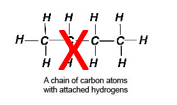
- All substances that contain carbon and hydrogen (-CH), such as sugar, are organic. Thus, they are not in the list of minerals.
4. It has an orderly internal structure
- The atoms, ions, or molecules are arranged in a repeating or orderly pattern known as crystal lattice.
5. It has a fixed (or uniformly variable) chemical composition.
- The composition and the percentage or proportion of the compositions of a mineral are fixed. This fixed chemical composition and internal atomic structure are reasons why minerals have specific physical properties and outward appearance. For example, quartz will always have the same percentage of silicon and oxygen.
PHYSICAL PROPERTIES OF MINERALS
1. Luster
reflect light?Is it metallic or nonmetallic? Is it opaque or transparent?

Metallic luster
- It has a shiny and opaque appearance when subjected to light. However, once subjected to air in a long time, it will become dull, so if checking the luster of a mineral, you have to check it through its broken part.
Nonmetallic luster -It has a transparent or translucent appearance when subjected to light. Examples of nonmetallic luster are vitreous luster (glass), dull luster (earthly appearance), pearly luster, greasy luster (oily), pearly luster, waxy luster (paraffin-like), and resinous luster (tree sap or resin-like.
2. Color
-A mineral's color is not considered a strong basis to identify its name because it could vary due to impurities.

For example, quartz appears in a different color because of its impurities or crystalline structure defect. This is the reason why you could find purple quartz (amethyst), colorless quartz (white), pink quartz (pink), and orange quartz (citrine).
3. Streak

4. Hardness
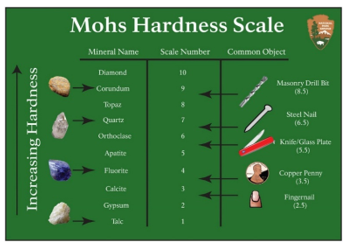
- It is the level or measure of a mineral's resistance to abrasion, surface friction, or scratches when it encounters other material. One standard way of measuring the hardness of a mineral is Mohs Hardness scale. It is designed by Friedrich Mohs, a German geologist. It measures the hardness of a minerals from a scale of 1 (softest) to 10 (strongest). The strength of a mineral is based on its ability to scratch another mineral (softer). Based on the scale, diamond is the hardest mineral (could scratch all minerals) while talc is the softest.
* Mohs Hardness scale is very easy to apply regardless of where you are as long as there is sufficient light. However, it cannot be used to precisely measure the hardness of industrial material.
5. Cleavage and Fracture (two ways on how a mineral breaks)
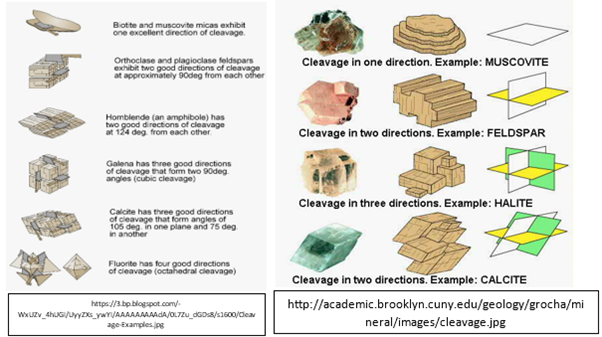
- Cleavage happens when a mineral breaks from a particular plane of weakness or where its internal atomic bonds are weak, forming a smooth, flat surface. The way a mineral cleaves is based on the structure of the mineral's component. If the mineral breaks in a different direction, the junction or the point where the breaks or cracks met is where the cleavage is. The parallel flat surface where a mineral breaks is called a cleavage plane.
- Fracture happens when a mineral breaks, not along a parallel flat surface, but in a random and irregular surface.
6. Crystal form
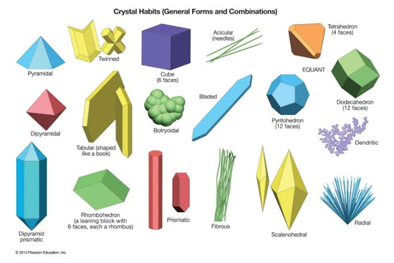
- It is the specific crystal structure or favored growth pattern of a mineral just before cleavage or fracture. This could be observed as the crystals grow in an open space. Examples of crystal form are given below. A mineral without a crystal structure is categorized as amorphous.
7. Specific gravity
- A particular mineral has a specific specific gravity, the ratio of the mineral density, and the density of water. You can use this to roughly identify or to base the name of an unknown mineral.
- There are various ways for you to identify the specific gravity of a mineral.
Specific Gravity = Weight of Mineral in Air/ (Weight of Mineral in Air - Weight of Mineral in Water)
Specific gravity= Weight of a Mineral/weight of water displaced by a mineral
Specific gravity= Weight of Mineral (in Grams)/ Volume of Mineral (in Cubic Centimeters)
Other Physical properties of a mineral
a. Magnetism- some minerals, particularly magnetites, are attracted to magnets because of the flow or movement of electrons in their crystalline structure. Minerals that are attracted to magnets are called paramagnetic minerals, while minerals that are not attracted to magnets are called diamagnetic minerals. 
b. Odor- these are minerals that release odor in a certain condition. For instance, sulfur smells like a lighting match at normal room temperature while it smells like a rotten egg when heated. Sulfides also smell like a rotten egg when heated. Arsenic smells mildly like garlic in a normal room condition, but the garlic odor becomes powerful when the arsenic is heated. This is similar with arsenopyrite.
c. Tenacity- this is the level of resistance of a mineral to bending or breaking. Halite, fluorite, and calcite are brittle because of their weak ionic bonds. Metallic minerals are malleable, gypsum, and calc can be cut into a thin sheet.
d. Reaction to acid- minerals such as carbonate minerals react with acids and release carbon dioxide. This is one way to identify a carbonate mineral.
e. Optical properties- it is how minerals behave when interacting with visible and invisible lights. Some minerals, such as gypsum, have fluorescence optical property (emit or release visible light when it absorbs energy from higher frequency radiation such as ultraviolet rays, x-rays, and electron beams). Minerals with phosphorescence optical property have similarity with fluorescence. However, it stores energy from the absorbed higher frequency radiation so it still emit light even if the source is removed. This allows the mineral to glow. Examples of phosphorescence minerals are calcite, celestite, colemanite, fluorite, sphalerite, and willemite. Thermoluminescence minerals glow when heated, such as apatite, calcite, fluorite, and lepidolite. glow when crushed, scratched, struck, or rubbed. Examples are calcite, felspar, fluorite, and quartz.

f. Radioactivity- there are minerals that produce radiation such as alpha, beta, and gamma. Examples of these minerals are Monazite (a mixed rare earth and thorium phosphate), Autunite (hydrated calcium uranium phosphate), Thorianite (thorium dioxide), Brannerite (uranium titanate), and Uraninite (uranium dioxide).
ELEMENTS THAT MOSTLY COMPOSE THE EARTH’S CRUST
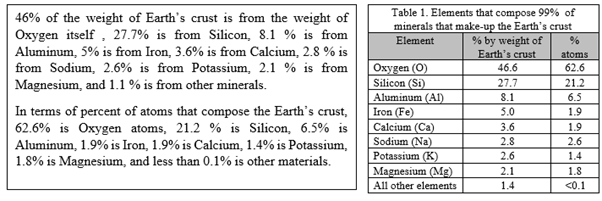
* These elements are also the ones that mostly compose the mineral components of the Earth’s crust.
CLASSIFICATION OF MINERALS
Minerals are classified according to the elements that compose them. For instance (check table 2 as you read this for your to know how to interpret the table), pure elements (Gold, Bismuth, and Diamond) that pass the qualification of minerals are under the category of native elements or minerals. A mineral that has silicon and 4 atoms of oxygen is under the category of silicate minerals. Examples of silicate minerals are quartz, olivine, and talc. Silicates compose 95%
of the Earth’s crust and upper mantle. A mineral (hematite, magnetite, and chromite) compose of 2 atoms of oxygen is under the category of oxide mineral. While an element mineral combined with halogens (fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)) is under the category of halide mineral. 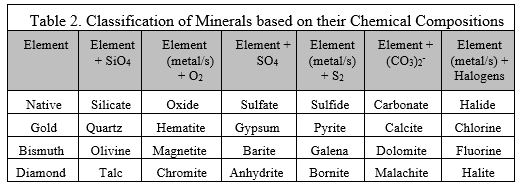
ROCK FORMING MINERALS
Three Conditions for a Minerals to be Classified as a Rock-Forming Mineral
Among thousands of minerals, there are only few rock-forming minerals. These minerals possess the following conditions:
a. One of the most abundant minerals on the Earth’s crust;
b. Original minerals present during crustal rock’s formation; and,
c. Important in classifying rocks.
Thus, rock-forming minerals are mostly made up of the elements that are abundant on the Earth’s crust.
Ten minerals that meet these criteria are:
a. plagioclase feldspars
- These are important minerals with a main composition of calcium and sodium. All plagioclase exhibit similar properties because of their similar crystal structure. These could be found in all rock types. These rocks have high resistance so even if they weather, their grains become a significant component of sediments, detrital, and sedimentary rock.
b. alkali feldspars
- These are silicate minerals with colored and glassy crystals. These are regarded as mixtures of potassium aluminosilicate (KAlSi3O8) and sodium aluminosilicate (NaAlSi3O8). They are abundant in acidic and alkali igneous rocks. Because of their properties, they are used to make ceramics and glasses. Alkali felspars that are highly colored, transparent, and iridescent are used as gemstones.
c. Quartz
- It is the second most abundant mineral in Earth's crust. It is composed of silicon and two atoms of oxygen (silicon dioxide). It occurs in a variety of colors (such as white, clear, gray, purple, yellow, brown, black, pink, green, and red) because of impurities, but its streak is colorless. It has a conchoidal fracture, glassy luster, hexagonal crystal system, and 2.6-2.7 specific gravity. It is used as abrasive, gemstones, and used in glassmaking.
d. Pyroxenes
- These are rock-forming silicate minerals that have a general composition of XYZ2O6. They exist in green to dark brown or black color but have a white streak. Pyroxenes have vitreous to dull color, have 3-4 specific gravity, and have a Mohs hardness of 5-7. They are utilized as industrial materials, and few of them are used as gem materials such as diopside and jadeite.
e. Amphiboles
- These are rock-forming minerals found in igneous and metamorphic rocks that have similar crystal structure and cleavage pattern. Amphiboles contain a different proportion of calcium (Ca), iron (Fe), sodium (Na), and magnesium (Mg). They exist in dark brown, dark green, and black colors but have a white to gray streak. They cleave in two directions, have virtuous to dull luster and opaque luster. Amphiboles' hardness is ranging from 5-6.
f. Micas
- Micas are silicate minerals that have a chemical composition of KAl2(Si3AlO10)(OH)2. Thick specimens appear to be black, silver, or brown, but their color becomes brown, yellow, green, or rose when split into thin sheets. They have a white streak, pearly to vitreous luster, perfect cleavage, 2.5-3 luster, 2.8-2.9 specific gravity, and a monoclinic crystal system. They are used in manufacturing cosmetics, drilling mud, joint compound, paint, and plastic rubber.
g. Clays
- Clays are sticky minerals when wet but coherent when dry with very tiny particles ( less than 2 microns) with flat sheets, slipper, sliding, and unique crystal structure. They become rocks when subjected to heat.
h. Olivine
- These are silicate minerals with a chemical composition of (Mg, Fe)2SiO4. Ca, Mn, and Ni rarely occupy the Mg and Fe positions. They exist in various colors (olive green, yellow-green to bright green, and brownish-green to brown) but have a colorless streak. There are olivine with vitreous luster and poor cleavage with a conchoidal fracture. They have a hardness ranging from 6.5- 7 and specific gravity ranging from 3.2-4.4.
i. Calcite
- There are minerals compose of Calcium and Carbonate (CaCO3), member of the calcite group. It exists in various colors (White, Yellow, Red, Orange, Blue, Green, Brown, and Gray) and luster (Vitreous, Sub-Vitreous, Resinous, Waxy, Pearly). They have very low hardness (3) with a specific gravity of 2.71. It is highly reactive with acids such as vinegar. Calcites are used as abrasive, construction aggregate, pharmaceutical, and construction material.
j. Dolomite
- Dolomites are rock-forming minerals that occur in different colors (pink, brown, pink, colorless, black) but have a white streak. There are vitreous dolomites and pearly dolomite. There are dolomites with perfect cleavage, rhombohedral, and those with that cleave in three directions. Their hardness is ranging from 3.5 – 4, and specific gravity ranging from 2.8-2.9. Dolomites are utilized in construction aggregate, agricultural soil treatments, dimension stone, and calcined to produce lime
CLASSIFICATION OF ROCKS
Since you are done learning the rock-forming minerals, let us jump into the different classifications of sedimentary rocks and relate it to the minerals that compose each of them.
1. Igneous rocks- rocks formed from solidified magma or lava. Magma are those hot molten materials that are found in Earth's mantle or below Earth's surface. Once this magma reached Earth's surface through volcano vents or volcanic eruptions, it becomes a lava. As this magma or lava solidifies, igneous rocks are formed. However, according to the location where the magma or lava solidified, there are two types of igneous rocks.
Two classifications of Igneous rocks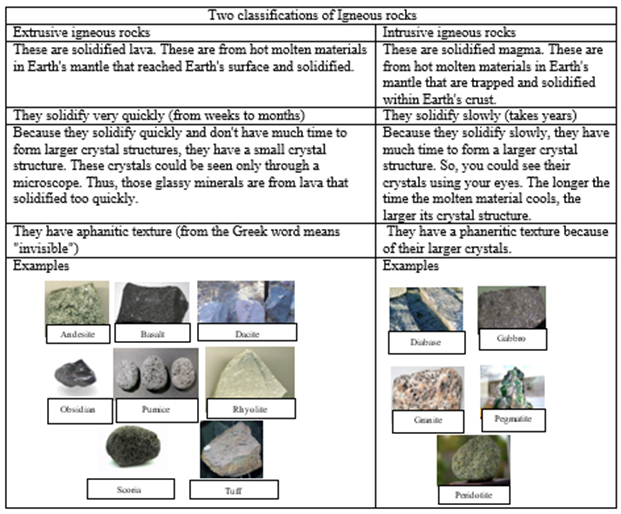
2. Sedimentary rocks
- Sedimentary rocks are types of rocks made from compacted and semented sediments. Sediments are solid materials, either a combination of rocks, parts of organic materials, or remains of living things that are settled on a surface and are compacted or compiled by different forces. The formation of this kind of rocks involves the following processes: weathering of rocks, sediment transport and deposition, compaction and cementation.
Two common features of sedimentary rocks
a. Strata (bedding or called lamination)'

- These are the layers observed in sedimentary rocks because of the change of their grains over time. Each layer usually represents different period.
b. Fossils
- These are the remains of dead animals or plants embedded or preserved in rocks.

Four types of Sedimentary rocks
1. Clastic Sedimentary
Rocks
- These are rocks that undergone various processes before becoming a sedimentary rock.
These processes are:
a. Weathering- It is the breaking of rocks into smaller fragments.
b. Erosions- It is a process in which the rock fragments or called as sediments, were suddenly transported due to either the pull of gravity, running water, by wind, or by moving ice.
c. Transportation- The process by which the sediments are transported by wind, running water in a stream, or other modes of transportation.
d.
Deposition- This is a process by which the force transporting the sediments is weak enough for the sediments to fall out and settle in one place.
e. Lithification (Diagenesis)- The process by which the sediments are compacted and cemented as materials that overlay them become heavier.
These are names of sedimentary rocks classified in terms of grain size and shape, among other factors.
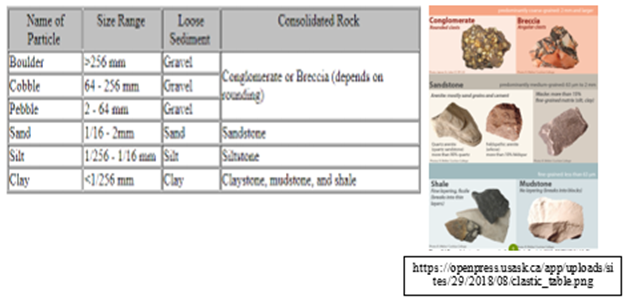
2-3. Biochemical and Organic Sedimentary rocks

- These are rocks that consist or are formed from the remains of living things. As organisms die, their remains can be accumulated and form sediments or sedimentary rocks. Examples of biochemical and organic sedimentary rocks are biochemical Limestone, biochemical chert, diatomite, and coal.
4. Chemical Sedimentary Rocks

- As rocks weather, they release ions. For silicate minerals, they usually release potassium, sodium, iron, calcium, and magnesium ions. Water carries these ions towards streams and underground. As these ions accumulate or its concentration gets too high, and as the water precipitates because of increased temperature, chemical precipitation happens. These allow the ions recombine, forming minerals. As these minerals accumulate and compacted, chemical sediments and chemical sedimentary rocks will form.
- Examples of these are Evaporites, Travertine, Dolostones, and Chemical Cherts
3. Metamorphic rocks
- This happens when igneous rocks, sedimentary rocks, or metamorphic rocks transformed due to metamorphism. Metamorphism is the change in chemical and physical structure of rocks because of its exposure to pressure, heat, and chemically active fluids. These commonly happen underneath Earth.
a. Contact metamorphism

- This type of metamorphism occurs when magma gets in contact with a rock. Its extreme heat causes rocks to transform chemically and physically. This usually happens in an
area where magma is intruding. The type of metamorphic rocks that are formed by this type of metamorphism is non-foliated metamorphic rocks. Non-foliated metamorphic rocks are metamorphic rocks that do not have a banded appearance or are not layered. Examples of non-foliated rocks are: marble (formed from the metamorphism of limestone or dolostone); quartzite; and hornfels (formed from the metamorphism of non-carbonate rocks).
b. Regional metamorphism (large-scale metamorphism)
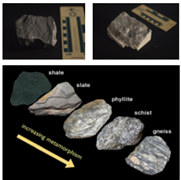
- This happens when great masses of rocks are transformed or changed due to high pressure. This usually happens to rocks near plate boundaries where tectonic plates diverge and converge, forming mountain builts and other features. The type of metamorphic rocks that are formed through this are foliated metamorphic rocks (banded or layered rocks).
- Examples of foliated rocks are skate, phyllite, schist, gneiss. Slate, when subjected to higher
pressure, can transform to phyllite. When phyllite is exposed to greater pressure, it can change to schist, and when schist is subjected again to greater pressure, it can turn gneiss. (check the image at the right side.)
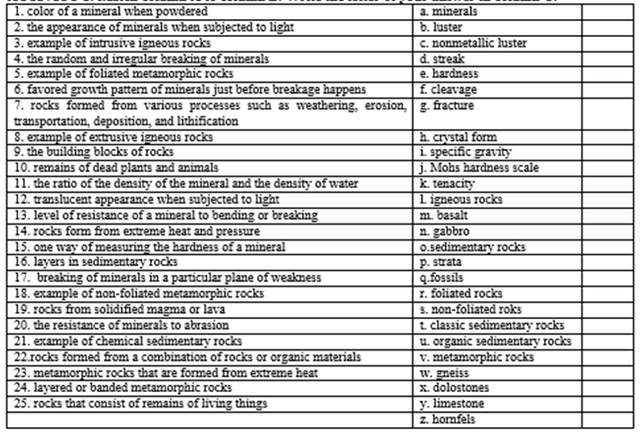
ACTIVITY
1: Match column A to column B. Write the letter of your answer in column C.
ACTIVITY 2: 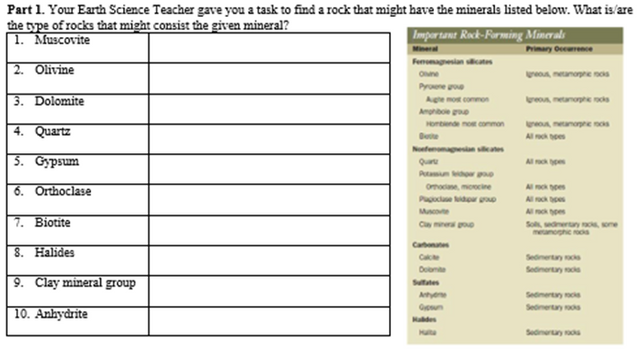
Part II. Analyze the rock cycle below and complete the statements or phrase that follows
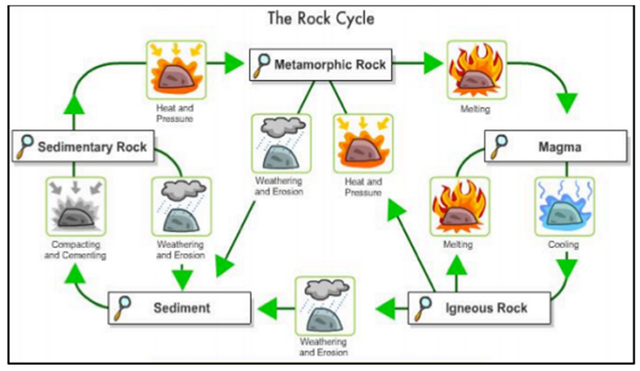
1. Sediments when compacted and cemented can transform into ______.
2. The part of an igneous, sedimentary, or metamorphic rock that weathered become ____.
3. A melted igneous rocks is called___.
4. Igneous rocks when subjected to heat and pressure become____.
5. A melted metamorphic rock is called____.
6. Sedimentary rocks when subjecte to heat and pressure become___.
7. Magma when exposed to low temperature become____.
8-10. Igneous rock can become a (___ ), (___ ), and (___ ) . It depends on the process or circumstance that the rock encountered.
ACTIVITY 3: APPLICATION
Part I. Below is an application of the use of some rocks in construction. Read the selection and answer the follow-up questions.
Stones are one of the primary materials used as construction materials. However, not all stones are utilized. The characteristics of rocks that are mainly used are durable, tough, durable, and are free from cracks and soft patches. Some stones are used as decoration or in minor construction because of their soft characteristic such as gneiss. While, some of these stones are used as construction materials such as basalts, granites, slate, sandstones, slate, laterite, marbles, quartzile, and travertine. Basalts are used in road construction, bridge piers, dams, and river walls. Granites are also used in bridge piers, dams, retaining walls, stone columns, railways, monumental utilizations, and external cladding of walls. Sandstones are used in bridge piers, dams, masonry works, and river walls. Slate is utilized in slabs, pavement, and roofing tiles. A compact, dense, and fine-textured type limestones are used in flooring, pavements, and roofing. Laterite is utilized as a building stone, but it needs to be plastered because it can easily be cut into blocks. Marbles are used as an ornament in columns, steps, and flooring. Quartzite is useful in building slabs, blocks, and as an aggregate of concrete. Lastly, Travertine is used for garden paths, paving, and courtyards.
Follow up questions:
1. What are the stones that are utilized in:
a. constructing railways
b. dams
c. roofing
d. bridge piers
e. flooring
ACTIVITY 4: GENERALIZATION
Instruction: Complete the statement below.
1. The five classifications of minerals are
2. Five of the most common rock forming minerals are
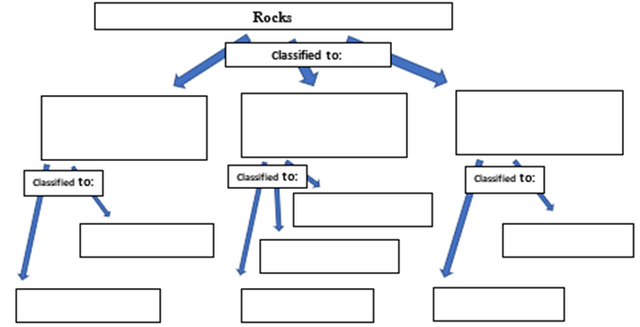
3. Complete the concept map below about the classifications of rocks
Part I. Analyze if the substance being described below is a mineral or not. If you think that the
substance is not a mineral, identify the physical or chemical characteristic that disqualified it as a mineral.
1-2. a naturally occurring organic compound with fix chemical composition
3-4. a liquid substance that has orderly internal structure
5-6. a man-made substance that has orderly internal structure, and fixed chemical composition
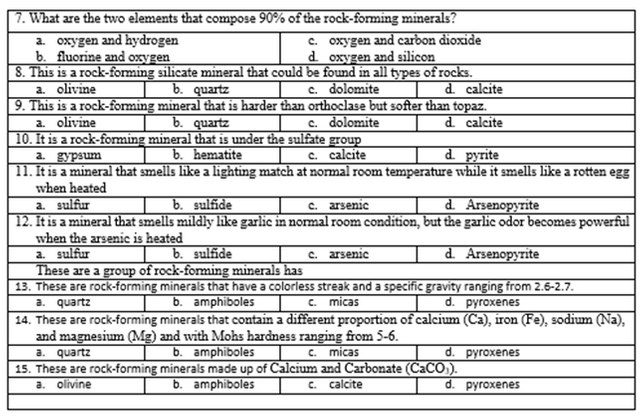
Part II. Choose your best answer from the choices and encircle the letter of your answer.
Part III. Classify the rocks below according to the type of rock where they belong.
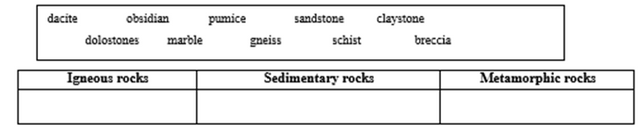
ACTIVITY 6. ADDITIONAL ACTIVITIES
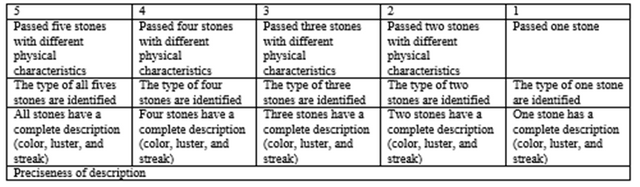
Collect five stones with different physical characteristics. Describe each stone's color, luster, and streak and identify an igneous rock, sedimentary rock, or metamorphic rock. You may place the stones in a clear plastic with a label and description attached in each. This will be passed together with your module next week.
REFERENCES
HARRY FRANK GUGGENHEIM HALL OF MINERALS, “Chemical
Properties of Minerals”, American Museum of Natural Resources, https://www.amnh.org/exhibitions/permanent/minerals/properties/chemical-properties-of-minerals
https://www.higp.hawaii.edu/~scott/GG101L/Jones2_minerals_chapters.pdf
The Editors of Encyclopaedia Britannicahttps://www.britannica.com/science/native-element, “Native element”, Britannica, 2020,
https://www.esci.umn.edu/courses/1001/minerals/plagioclase_feldspar.shtml
https://www.britannica.com/science/alkali-feldspar
https://geology.com/minerals/quartz.shtml