Introduction
Quantum Mechanics is the basic theory that describes the atoms and elementary particles, how they move around and what they might look like. It was invented in a couple of decades in the 20th century by many scientists working together like Heisenberg, Plank, Einstein, Schrodinger and many more.
At this point, these guys already knew a lot about atoms. That they have a nucleus that consists of protons and neutrons and that electrons are somewhere around this nucleus. (Click here to read more about the discovery of a nucleus) They understood that the neutrons and protons are clumped together in a very tiny space (Nucleus 0.01 picometer). And the rest of the atom (around 300 picometers in diameter) is mostly empty space with electrons.
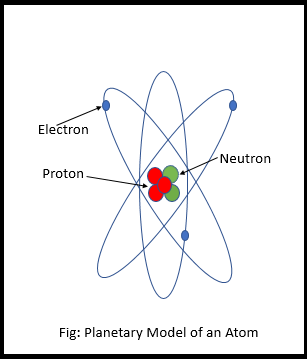
The Planetary Model of the Atom
But they didn’t know exactly where the electrons are, how they move around, or what path they follow. So they made an initial sketch of the atom, with the core at the center and the electron revolving around it. They called it the planetary model of the atom (Picture on the side).
It was proven to be wrong but it was a starting point, which allowed scientist to do the research and experiments.
The dual nature of electrons
One of the Physicist noticed that when the electrons were forced to interact with matter (by hitting a detector with the electron), the electrons behaved like a particle (obviously). But when the electrons were allowed to move freely and undisturbed, they always acted like a wave. (The dual nature of particle can also be observed by the "Double Slit Experiment" but more on that later.)
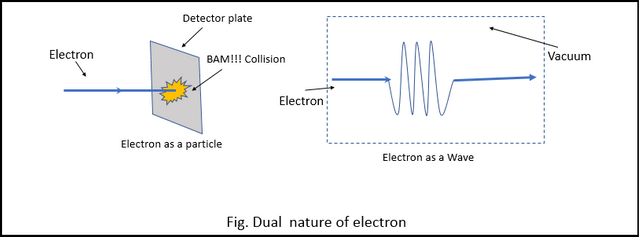
This was the first time scientists came across particle-wave duality. and it threw the Planetary model out of the window. They now needed a new model, but they knew the model couldn't come from classical mechanics. They needed a whole new kind of mechanics, a theory that includes the duality of the particle.
The Schrödinger Equation
Enter Erwin Schrödinger (Yes, the guy who owns an imaginary curious cat). He presented the world his famous wave equation which was the rock-solid core foundation upon which quantum mechanics was built.
THE SCHRODINGER EQUATION, the equation itself needs a separate article to be explained.
Maybe I'll do an article for Schrodinger equation in the future, but for now, let's move on.
The equation below is Time-dependent Schrödinger equation (general)
(Don’t panic, it's just one equation)
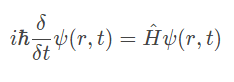
The equation is able to determine the probability of finding an electron across the wave function.
So when we put the various different variables for the particle, say the electron of a hydrogen atom, the equation spits out the location of the electron for the instance.
The shape of the atom
When we repeat the operation with different values, combining the values gives us this beautiful shape of the atomic orbitals in which the electron is probable to exist. A hydrogen atom has the atomic number 1, which means it has one electron (in the 1S orbital), and the nucleus consists of one neutron and one proton. So, the image we get for the hydrogen atom looks like the first picture of the s orbital below. And for the atoms with a higher atomic number than 1 are subsequently arranged in the different types of orbitals. The first three types of orbitals are shown in the picture below.
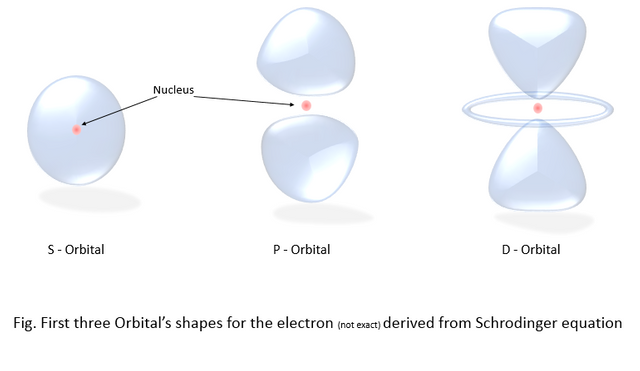
Tell me that’s not beautiful, The red ball in the middle is the nucleus and the blue blob shaped thingy is the cloud of probability for the location of the electron. That means the electron is roaming around the whole cloud all the time until it is observed. And when we take the measurement to find the location of the electron, the whole probability cloud collapses into one point. The phenomenon is better explained by "Heisenberg's Uncertainty principle".
I'll definitely explain the Heisenberg's Uncertainty Principle in my future posts. (Spoilers: The Heisenberg we're talking about is not Walter White.)
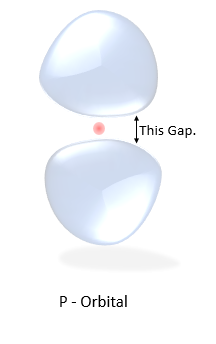
The Anomaly
Notice the probability cloud in the orbital P.
They are not connected, there's a gap between them. The electron cannot exist in the gap.
(Picture on the right)
there's zero probability for the electron to exist there. That creates the question how on earth the electron can travel from one part of the blob to another. Is the electron teleporting, is it skipping the space somehow, what's going on here?? But the problem was later explained using Bell's Theorem.
This long debate stayed on for 40 years which led the Einstein to say the quote below.
"I, in any case, am convinced He does not play dice with the universe."
-Albert Einstein(1926)
Source & References: Wikipedia, Introductory Quantum Mechanics by Richard Liboff 1987
Image Source: All Self-created.
I would also recommend watching this youtube video by SciShow.
Special thanks to @suesa for accepting me as a mentee in her Mentoring program & Helping me write this article. Click here to know more about Suesa's Mentoring Program

Daily Fun Fact #17 -- "Sea otters hold hands while sleeping so they don't drift away from each other."
Mmmm sweet memories of the first year as the student and the course of physical chemistry
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Great to see you doing well man :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
All Thanks to you, you believed in me & gave me a chance :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
very interresting thank you
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thank you for taking interest.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Can you explain Field Theory?
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I'd love to, maybe in the future posts. :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @arrjey, this post is the third most rewarded post (based on pending payouts) in the last 12 hours written by a Dust account holder (accounts that hold between 0 and 0.01 Mega Vests). The total number of posts by Dust account holders during this period was 3389 and the total pending payments to posts in this category was $448.91. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit