Today I would like to take a look at an article from the American Chemical Society (May 2017) describing a very interesting concept: Bringing water to the desert or having increased cooling power in cooler units using Smart Chemistry.
Authors Rieth and Dinca, from MIT describe their work as follows:
The capture of water vapor at low relative humidity is desirable for producing potable water in desert regions and for heat transfer and storage. Here, we report a mesoporous metal–organic framework that captures 82% water by weight below 30% relative humidity. Under simulated desert conditions, the sorbent would deliver 0.82 gH2O gMOF–1, nearly double the quantity of fresh water compared to the previous best material. The material further demonstrates a cooling capacity of 400 kWh m–3 per cycle, also a record value for a sorbent capable of creating a 20 °C difference between ambient and output temperature. The water uptake in this sorbent is optimized: the pore diameter of our material is above the critical diameter for water capillary action, enabling water uptake at the limit of reversibility.
But what does that mean?
I'll try to explain further. The team developed a metal organic framework (MOF) that is very hydrophilic, so that even in a desert it would pick up water!. You may use it for drinking water, or use it in an air cooling unit.
It only works at night, while then the humidity is higher than 28% (is still very low). Then, it is possible to collect almost 90% of its own weight.
At daytime, when it maybe up to 45 degrees C and a 5% humidity the MOF releases the water automatically. So, easy to catch.
The Chemistry
The pores are defined by one-dimensional chains of five-coordinate metal atoms with hydrophilic open coordination sites supported by strong metal–azolate linkages, see the picture.
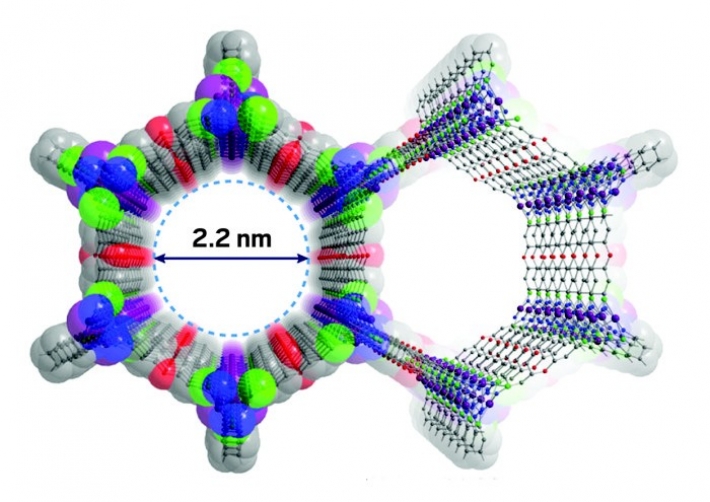
Figure 1: a picture of the metal organic framework, which is built from metal ions and organic clusters. They are a sort of polymers, functionalized with metals. They have Pores, where reactions can take place, or where other molecules fit in. This MOF is designed to fit exactly a water (H2O) molecule.
By changing metals combinations, different pore volumes and sizes are reached. If you are able to select the right atom elements, the H2O molecule will fit!

Depending on the metals used, you clearly see a different adsorption pattern!

Graph explanation: Top picture: Water vapor adsorption (closed symbols) and desorption (open symbols) for the different tested MOFs. Bottom picture: (B) Comparison of MOF and zeolites investigated for water sorption. Materials that take up water between 10% and 30% RH are desirable for their strong affinity for water and their relative ease of regeneration. In the graph you see that MOF 2 of the authors from MIT has the best water adsorption. It is even twice better than the currently known "best one".
The experiment in practice!
Here you se a demonstration of the MOF in the desert conditions: At night time it catches water, at daytime it s automatically released.
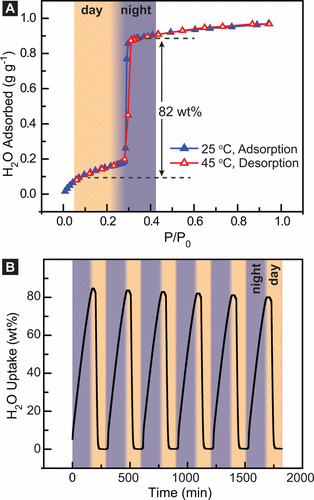
Graph explanation: You are looking at the repetitive cycle of adsorbing and releasing water. It is very important that the authors prove that that works cycle after cycle! It is really working. That is *"Proof of the Hypothesis" *
Do you want to learn more?
visit the original article at : http://pubs.acs.org/doi/full/10.1021/acscentsci.7b00186#showFigures
Info about Metal Organic Frameworks: https://en.wikipedia.org/wiki/Metal-organic_framework
Sources / Reference (article and pictures)
http://pubs.acs.org/doi/full/10.1021/acscentsci.7b00186#showFigures
This post received a 1.7% upvote from @randowhale thanks to @beesteem! For more information, click here!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Nice job! You could give a bit more detail about how to interpret the plots (I have no trouble but non scientists may not know exactly what they are looking at, this is an aspect to writing that I am still working on too), but still great post.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Great, thanks. I will add some more info coming minutes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
done :-)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Awesome, this is much improved :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit