Build your own Biosensor
Development of a Biosensor for glucose detection
Background
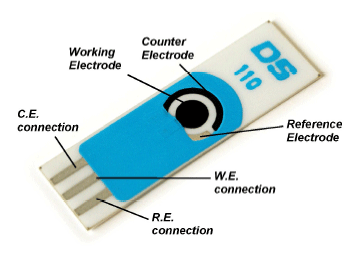
I had some time over the other day, and some glucose oxidase to spare so i decided to design 4 x glucose biosensors for fun, since I had all the needed ingredients. I pulled out an old recipe and design schedule, and made a 1st generation glucose sensor from scratch on a DS 110 carbon diode electrode with an Ag/AgCl counter electrode. The sensor is based on Prussian blue, glucose oxidase and some usage of cyclic voltammetry/amperometry. I chose a cheap and easy to replicate design so you can try it yourself.
I will provide the full list of ingredients and a detailed design plan that anyone can follow!
p.s all the images and facts with no references are my own design and I give full rite of usage to everyone in any context.
I measured the sugar content in what I could get my hands on around the laboratory, which was coffee, coffee with sugar, soda, diluted soda and some PBS(who doesn't have that doh)
The first design worked well and here is the data from my trail run. It took me about 4 hours to set up 4 x sensors due to using cyclic voltammetry and the time it requires.
What is biosensors, and what is the glucose sensor used for?
The definition of a biosensor is a sensor for a given analyte, base on biological active system produced in vivo or synthesized to the same structure and function. The biological system creates a unique signal corresponding to the analyte we wish to measure. In the sensor I designed, we measure the amount of hydrogen peroxide that is created by glucose oxidase when it catalyzes the reaction of glucose into gluconic acid. We do this via a catalytic reaction with Prussian blue, containing redox active copper & cyanide ions, which further catalyzes the reaction of hydrogen peroxide and changes the copper & cyanide complex structure and charge. To be precise, It is not a redox mediator, it is a catalytic biosensor. We can trace the amount of copper that changed state exactly via the current we measure over the electrodes, making the sensor pretty precise when manufactured to satisfaction.
The most common use for the glucose sensor is off course in medicine and especially towards diabetic patients who need to know their level of blood sugar, at home, continuously.
Blood-glucose biosensors usage at home accounts for 85% of the gigantic world market.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862100/
There are many different properties besides electrochemical ones( like the redox mechanism in our design) that can be used to design a biosensor. It can be acoustic , magnetic or optical signals that change due to biochemical events. There are many uses besides the medical industry and the first that comes into mind is the food industry. They need to monitor bacteria and chemical pollutants at a production stage. During the transportation / selling periods, the food is randomly tested for freshens via trace chemicals, all this can be done in a cheap and time saving manner with biosensors.
Fermentation procedures are sensitive to many different changes in chemical composition, and requires some monitoring. Biosensors make it easy to measure e.g oxygen or yeast concentration in a solution or test for unwanted metals / bacteria.
An arduous quandary in food processing industry is of quality and safety, maintenance of food products and processing. Traditional techniques performing chemical experiments and spectroscopy have shortcomings due to human fatigue, are expensive and time consuming. Alternatives for food authentication and monitoring with objective and consistent measurement of food products, in a cost effective manner, are desirable for the food industry. Thus development of biosensors in response to the demand for simple, real-time, selective and inexpensive techniques is seemingly propitious.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862100/
Principle of our glucose biosensor
There are three common types of generations of biosensors, 1, 2 and third generation biosensors which differ in signal transduction. The different generations of sensors can be seen in the second picture from the top.
An electrochemical glucose biosensor was developed, with a first generation design adding a catalytic element. It consists of three electrodes; a working-, counter- and reference electrode. The potential is measured between a working electrode and a reference electrode. In contrast, current is measured between a working electrode and a counter electrode. The techniques cyclic voltammetry and amperometry was used to activate, functionalize and validate the developed biosensor.
The working principle of the biosensor can be seen in Figure 1 and is based on the formation of electroactive molecules. Detection of D-glucose is based on activity of glucose oxidase in the reaction;
Hydrogen peroxide (H2O2) interacts with a ferric ferrocyanide in the redox reaction;
This reaction produces an anodic current which is measured between a working- and counter electrode.
Ingredients and laboratory setup
The recipe is common and I used it as a student as well, when taking the course in biosensors, hope you find it usefull.
To send currents between E.g. -0.7 and 0.7 volt, and sweep the range with small increments at a time while measuring the current, you need software and some kind of electrode socket box connected to a potentiostat.
I used PalmSens software PTStrace 5 for windows(they also have electrodes and potentiostat) which will be visible throughout the design, the hardware is shown in the picture above.
This is the actual recipe and setup I used so it sure works. If you have any questions about acquiring them or doing dilutions or anything biochemical / laboratory really, leave a comment
The main problems with reproducibility is:
- pH, sensitive, keep constant and physiological.
- Temperature, sensitive, must be kept constant.
- Dynamic range, the largest and smallest possible measurable value, might change somewhat, making it very calibration sensitive.
Step by step design tutorial
This is the actual data from my design, if you try the procedure you should be able to replicate the pattern, no matter the circumstances. I had to use print screen for the captures from PalmSens/PTStrace 5 software, due to the fact that our laboratory computers never connect to the internet or internal net and lacks all software expect the designated one, it came down to this.
- Preparation of enzyme-nafion electrodes as followed:
- Place a droplet ~1 µl of the enzyme solution cover all the Prussian blue functionalized electrode surfaces (Reminder: Only place the enzyme/nafion
solution onto the WORKING ELECTRODE surface). Then, keep the electrodes
at room temperature for 15 minutes to dry. - Place a droplet ~1 µl of the nafion solution onto the electrode surfaces covering the
all the enzyme layer. Then, keep the electrodes at room temperature for 5 minutes to
dry
5. The last part is about calibrating the sensor to stock solutions of glucose, which is easily prepared and diluted after your needs.
6.Measure away!
- Place a drop of your beverage on the electrode as regular.
- Chose amperometric response with settings like step 3 and 5 then measure the sugar content in soda, coffee or whatever you want to measure. I would also suggest to design 10 of them while you are at it, since there are some troubles with gaining reproducibility in the results when using this method.
- Diluting the samples can help improving measure quality!
- Done!
The results are not bad, the lower beverages with low sugar content clearly separate and in the right order.
The more sugary samples is less stable, since the reaction rate can´t be expected to be the same all the time, after the bottom layer is depleted from glucose the signal might get a bit unstable. The medical grade sensors have much more precise delivery mechanisms and thereby much greater sensitivity and takes smaller samples as well.

wow, what a response. 143 upvotes. You hit a diabetic nerve
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Try the procedure :))
Thanks for your support :) Peace
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Quite thorough, high-level stuff.
Now if you design me a complete home blood test kit, that would save me some money!
:P
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Well if you had an optimal sample as reference, I think this design could detect a level that is lower, prior to your death :)) but I would´t bet anyone´s life on it, so I ll have to get back to you on that hehe
Growing insuline in E.coli is easier at home I´d say and saves more money perhaps?
Peace
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I have never done CV in aqueous solution before... any tips or tricks to get them to look that nice in that medium?
Its sufficiently tricky for me remove oxygen (via purging with nitrogen) from anhydrous organic solvents as it is for CV sometimes.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hey, I think it is preferred to use aqueous solutions, since it is more flexible, I haven´t tried the other way around, since more specific combinations is needed.
All i know about this topic in organic polar solvents is that we use NBu4PF6, for its inter properties, and I am specialized in proteins sorry ;) So I would rather start at one of these points, then to purge another composition of solvent. Keep pH and temperature exactly stable, so the O2 content is always the same. Use low scan rate ^^
Look at the result screen, It´s not that good! The rest of the Images are cleaned or produced in steps where they always look decent ;)
But if you try a design I got experience from i could point to some problems of reproducability in more detail then the text!
Thanks for reading!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
very good post my friend like your post, my resteem and upvote yes
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks for checking it out!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Being A SteemStem Member
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Wow, thanks for this post. I'm working on my chemistry bachelor right now, this is a great content!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
My pleasure :) Thanks you for checking it out!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Really cool!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Biochemistry in action :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You just planted 0.70 tree(s)!
Thanks to @peach023
We have planted already 7728.41 trees
out of 1,000,000
Let's save and restore Abongphen Highland Forest
in Cameroonian village Kedjom-Keku!
Plant trees with @treeplanter and get paid for it!
My Steem Power = 18425.67
Thanks a lot!
@martin.mikes coordinator of @kedjom-keku
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Clausewitz from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit