Anomalies of Water
The study of the most abundant compound on earth-water has had a long history. In the first millenium BCE Thales of Miletus statement that:
The principle of all things is water
- Thales from Miletus
And guess what? He was right. Water has remarkable physical and chemical properties, some of which are anomalous.
What is anomalous liquid?
Anomalous liquid is define as a monocomponent substance that, in the liquid phase, behaves generally diffrent in respect to the argon-like liquids. In case of water these atypical properties are given by unique features of individual water molecules and they are attributed to the presence of an extensive hydrogen-bond network.
Water phase anomalies:
1) Unusually high melting and boiling point.
1.1. Water - molecule structure
If u consider the atomic composition of water, u should assume that water itself in normal conditions should be gas. Also its liquid rage would be somewhere between -80˚C and -110˚C not between 0˚C and +100˚C. Especially when compared with its heavier analogues from lower periods of periodic table: sulfane, selane, and tellane.

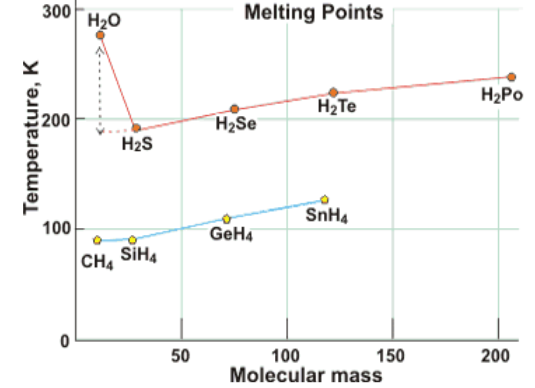
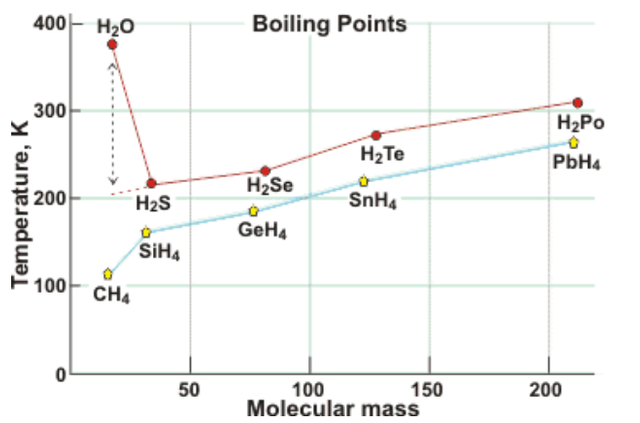
1.2.Comparison with other hydrides
Melting/boiling points of hydrogen sulfide/selenide/telluride are -82°C/-60°C; -65,73°C/-41,25°C and -49°C/-2,2°C respectivelly. Water is a light molecule (H2O (18)), it should require less energy to boil than H2S (34), H2Se (81), H2Te (130) while the water boiling point is over 150K higher than expected by extrapolation of the boiling points of other group 6 hydrides. Also melting point of water is over 100K higher than expected by extrapolation.
1.3. Explanation
When comes to water anomalous high temperatures can be explain by the strong associations between water molecules so-called hydrogen bonds. Even thought hydrogen bonds are weaker than covalents bond, to melt or boil the water we need break a lot of these. To achive this we need a lot of energy.
Tab. 1. Liquid range and hydrogen bond energies of several liquids.
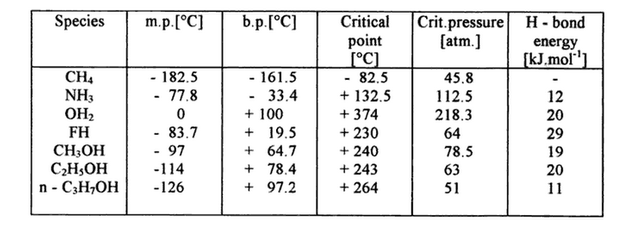
But hydrogen bonds can occur in others liquid too. Moreover hydrogen bonding is stronger in liquid hydrogen floride than in liquid water. So why is it melting and boiling point are considerably lower?
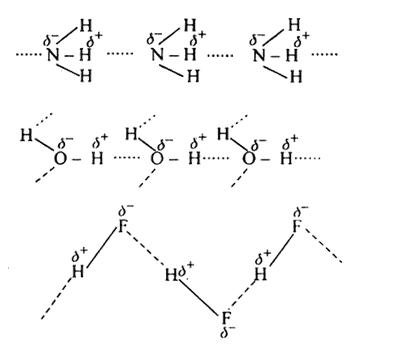
The crucial difference between liquid H2O and liquid HF is the different structural arrangement. HF has three lone pairs on the F atom but only one H atom- can form only two hydrogen bonds (one-dimensional association) while each H2O molecule can form even four hydrogen bonds (three-dimensional network). This 3D network is extremely strong and yet extremely flexible and adaptable.
Well done! The next step should be to explain why water can solve so much other HnXn-components and that´s why is a very universal solvent.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit