During the body's typical healing process, when tissues like skin are damaged the body grows replacement cells. Integrins are class of proteins that are important in the cellular processes critical to creating new tissue. One of the processes is cell adhesion, when new cells "stick" to the materials between cells, called the extracellular matrix. Another is cell migration, where at the cell's surface, integrins help "pull" the cell along through the extracellular matrix to move cells into place. However, these processes do not occur in brain tissue that has been damaged during a stroke. This is why scientists are trying to develop therapeutic materials that could promote this form of healing.
The injectable gel-like material, which is called a hydrogel, that the UCLA researchers developed helps this repair process by forming a scaffold inside the wound that acts as like an artificial extracellular matrix, and the new tissue grows around that.
Using an injectable gel isn't new, but previous gel scaffolds resulted in weak blood vessels in the newly formed tissue. The new findings, published in Nature Materials, show that when the scaffold contains a specific integrin-binding molecule, the new blood vessels that are formed are stronger.
"The injectable gel scaffold is sort of like a garden trellis that plants use to grow on," said Tatiana Segura, professor of chemical and biomolecular engineering, bioengineering and dermatology, who led the research. "By itself that's good as incoming new tissue has something to support its growth. This new material is similar to a trellis with very specific fertilizer to help the plant grow healthy and strong."
Even combining gels with a protein that promotes blood vessel formation, such as vascular endothelial growth factor, known as VEGF, the blood vessels in the new tissue inside the scaffold tend be leaky and also tend to clump too close together.
To overcome this, the researchers looked deeper at how integrin-binding molecules interacted with the gel and how these molecules influenced blood vessel growth.
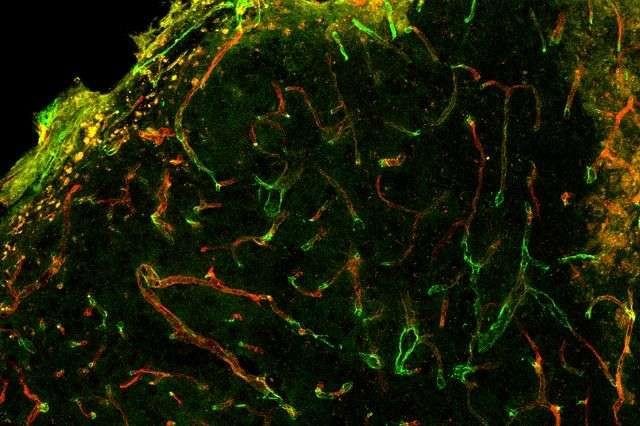
They tested two types of scaffolds with different integrin-binding molecules. Both scaffolds also contained the VEGF protein. They found that one of the scaffolds—which bound with the integrin known as "α3/α5β1"—worked really well. It directed a higher quality of repair and of regeneration of blood vessels. In addition, they found that the α3/α5β1 binding scaffolds also guided the shape of the blood vessel, a process called morphogenic signaling.
The other integrin-binding scaffold they tested still had problems with leaky and clumping blood vessels.
"Beyond just the structural support for new tissue and blood vessels, the addition of specific integrin binding molecules for α3/α5β1, tells surrounding tissue to grow blood vessels that are strong and well-defined as opposed to other one we tested, where new blood vessels were prone to leaks and clumping too close together," Segura said.
The lead author on the research was Shuoran Li, a 2017 UCLA doctoral graduate who was advised by Segura. Collaborators also include Dr. Thomas Carmichael, a neurologist and neuroscientist at the David Geffen School of Medicine at UCLA and Thomas Barker, a professor of biomedical engineering at the University of Virginia.
In this recent work, the researchers demonstrated that integrin binding can dictate blood vessel structure in vitro with the α3/α5β1-binding scaffold resulting in extensive networks that link up with existing blood vessel branches. Then the researchers used the same α3/α5β1-scaffolds in mice and saw blood vessels formed that were less leaky following stroke.
The next step, the researchers said, would be using integrin-binding molecules with other hydrogel technologies that have shown promise for long-term functional recovery after stroke, but in which newly grown blood vessels were not robust.
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://phys.org/news/2017-08-gel-wound-healing-material.html
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit