
Although meteorites and rocks are the main focus of my collection, I also like to collect trinitite. It may represent a small part of my overall collection, but it is a part that is very interesting and historically-significant. Here, I will answer many of the common questions I get from other collectors and the curious.
Q - What is trinitite?
Trinitite is a strange, green, glassy substance that was created when the US military detonated the world's first nuclear bomb at the Trinitity Test Site (now White Sands Range) during World War II in 1945. The atomic blast vaporized the device casing, the gantry tower, and the surrounding desert sand into molten green blobs which then fell to the ground and solidified as trinitite. This glass is also known as atomite and atomsite.
Q - Are these trinitite specimens genuine?
Yes. The source I acquired it from bought out the supply of an old New Mexico rock shop that went out of business in the 1970's. This rock shop acquired it directly from the Ralph Pray stock via Verne Byrnes. Trinitite would be difficult to fake and it is not a very profitable collectible, so there is no reason to offer bogus trinitite - there are much more valuable items that could be forged more easily than trinitite. I have had two reputable scientists test my trinitite samples (for their own studies) and this material is the real deal. You can read the full story about the original source of trinitite at this link - http://www.mine-engineer.com/mining/trinity.htm
Q - Is trinitite legal?
Yes. This trinitite was removed from the Trinitity site many decades ago before any laws or controls were in place. As such, it is "grand-fathered" onto the market and is 100% legal to own. Many years ago, the government sealed off the site and the removal of trinitite is now forbidden by law, so you cannot go there now and pick up your own.
Q - Is trinitite safe?
Yes, for the most part. Trinitite contains an exotic mixture of trace compounds and elements, including tiny amounts of plutonium isotopes. However, the quantities of these dangerous elements are extremely small, and the most toxic compounds have degraded long ago into less-harmful decay products. You can own trinitite, display it, or handle it and it will not harm you. Just do not break the trinitite up which may release dust or small particles. Do not eat or inhale the trinitite. And keep it away from children or pets who may eat it. Wash your hands after handling it. Stored and handled properly, it is completely safe.
Q - Is trinitite radioactive?
Yes. It is mildly radioactive, but at safe levels. To put the radiation hazard into perspective - you get more radiation from standing outside in the daytime and your smoke detector is more radioactive than trinitite. The radioactivity will measure in very small amounts on a geiger counter, depending on how close the sample is to the instrument probe.
Q - What are the different forms of trinitite?
The vast majority of trinitite is a pale-green material that is rough and bumpy on one side, and smooth on the other side. The shade of green can vary slightly from light to dark. There are also pieces of reddish or orangish trinitite that is thought to get some of it's coloration from copper and iron in the device casing and gantry tower. Black trinitite is very rare and is never seen on the collector market.
Q - Can I get a BIG piece of trinitite?
Not easily and not from me. Nearly all trinitite specimens on the collector market consist of small, irregular-sized fragments ranging in size from less than a gram to about 8 or 9 grams. Anything bigger than 10 grams is considered increasingly rare and difficult to acquire. Because trinitite is porous and light, the pieces tend to be quite big for their weight, compared to denser materials like rocks or meteorites.Also, most trinitite is somewhat brittle and larger pieces tend to break.
Q - Why collect trinitite?
Trinitite is a unique historical collectible that represents mankind's mastery over the atom. Trinitite symbolizes technological achievement and the beginning of the Cold War and nuclear arms race. From a mineral collector's standpoint, trinitite contains a large number of exotic elements and minerals in trace form.
Q - Will trinitite give me superpowers or turn me into a mutant?
No, it will not. ;)
Q - Where can I buy trinitite?
Here - http://www.galactic-stone.com/products/trinitite

Further information about trinitite :
Each piece of trinitite is unique. The majority of trinitite specimens share these characteristics :
Typical size is about 2 to 7 grams. Pieces larger than 7-8 grams are uncommon. Pieces larger than 9-10 grams are quite rare. Pieces over 10 grams are very rare. This is because trinitite is brittle and was broken up during the process of harvesting it. Repeated handling, transport, and storage over 50+ years has further broken up the material. The vast majority of trinitite specimens on the market are about the size of a US quarter.
There are two distinctly different sides to most pieces. A smooth, glassy, green side, and a bumpy beige side. Right after the blast, while the trinitite was in a molten state, it solidified on the desert floor. The bumpy beige side was facing down into the sandy desert ground. The smooth green side was facing upwards to the sky. During the cooling and hardening process, a coating of sand and tiny pebbles was adhered to the side of the trinitite facing down into the ground, while the side facing up remains free of debris. The bumpy side emits less residual radiation, because the coating of sand and pebbles absorbs much of the alpha and beta radiation emitted by the trace isotopes contained in the trinitite.
Pieces with broken edges will show a highly-vesiculated interior with a glassy appearance and green coloration. Tiny black specks or other pinpoint-sized inclusions are not uncommon. Tiny flecks of metal are very rare. These tiny flecks of metal may be remnants of the atom-bomb casing, gantry tower, and/or atom-bomb components.
Most pieces emit very little radioactivity. Testing with a geiger counter shows a uniformly low level of alpha, beta, and gamma emissions. Exact levels of activity vary from specimen to specimen and depend in part on how far each specimen was from ground zero during formation. The bumpy side of a given piece shows a lower level of activity because the sand and pebbles on the exterior surface absorb some of the alpha and beta emissions.
The predominant color is green, ranging from light olive-drab to brilliant emerald, and all shades in between. Red is less common. Black is very rare.
Most shapes are blobby and irregular, but thin in profile. Most pieces are no more than 3-5mm thick. Occasionally, thicker pieces are seen, but they are much less common.
Safely displaying your trinitite :
Note, the radioactivity level of most trinitite is very minimal. It is not dangerously radioactive. In fact, your smoke detector is more potentially dangerous than trinitite.
But, radioactivity is to be respected, so here is a tip for displaying trinitite to minimize radiation exposure.
Trinitite contains a mixed soup of radioactive isotopes and daughter products. It emits varying levels of radiation in alpha, beta, and gamma.
If you store your trinitite in a riker box display (like the ones I sell), put the bumpy/ugly side of the trinitite outwards facing the glass. Why?
The bumpy side is coated with a layer of sand and tiny pebbles that were fused to the surface of the trinitite during formation. This is the side of the trinitite piece that was facing the ground while molten. The smooth glassy green side was facing up during cooling.
So, the bumpy sand and pebbles act as a barrier to stop some of the alpha and beta radiation. I have confirmed with a geiger counter that the bumpy side is less active and emits considerably less radiation than the smooth green side.
Inside a riker box, the glass viewing window of the lid blocks almost all of the remaining alpha and beta emissions that do penetrate the bumpy sand/pebble coating. There is virtually no detectable activity outside the glass, even if the geiger counter is held right up against the glass.
The backside of the specimen is still emitting, but it is radiating from the reverse side of the display box. If you put the box on a shelf, with the back facing a wall, then you have reduced your level of exposure to zero or almost zero. Also note, the stiff cardboard and foam filler of the riker box back does not block radiation as effectively as the glass lid does. So a geiger counter will show activity (reduced) if held to the back of the display, and will show almost no activity at all from the front.
If you keep a smaller specimen in an acrylic gemjar, then the gemjar itself will block almost all of alpha and a portion of the beta.
Note, gamma radiation will penetrate glass and acrylic readily. Thankfully, trinitite does not emit strongly in gamma.
Between the small size of most trinitite specimens, and the low level of activity, your exposure level to a single piece of trinitite is very very small. If stored and display properly, it poses the same exposure risk as having a smoke detector mounted on your wall.
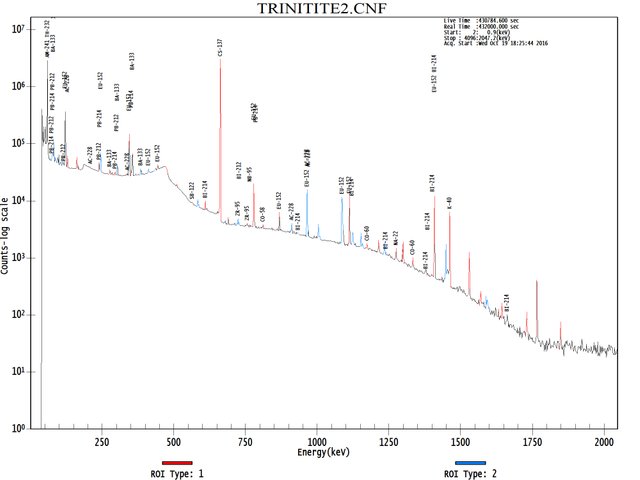
Image below : Isotope analysis plot. Testing done at Penn State, Geo Sciences Lab. (Open image in a new tab to see the full-size version).
Weird, just did a posting myself on the Manhattan project.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
http://galactic-stone.com/trinitite-faq/
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks. I am the author and that is my website. :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit