Introduction

Humans are already prone and exposed to the changing temperatures present on Earth. How we react to certain temperatures clearly signifies that we are still alive. Moreover, every thing under the sun- be it living or non-living has its own way of reacting to certain temperatures. Even so, in determining temperatures we used the thermometer to know precisely the temperature reading in a much clearer sense. Thermometers are simple scientific instruments based on the idea that metals change their behavior in a very precise way as they get hotter (gain more heat energy). We already know since our childhood that the red liquid which is actually put inside a thermometer, rises when its hot but tends to drop when its cold.
But how does that red liquid react to temperatures? What's really the phenomenon behind?
During elementary classes, we were already taught that metals are conductive- reactive too. Yes! When applied with varying temperatures, they tend to react in such a way that their molecules move apart or closely which causes vibration due to expansion. One of such phenomenon is called thermal expansion which will be discussed on the proceeding topics. This blog offers you an insight about how that red liquid Mercury (Hg) reacts to certain temperatures; what's the reason why of all liquids, it's used to be put inside a thermometer; why when heated it goes up and when cold goes down.
What is Mercury?

Mercury is a chemical element with symbol Hg and atomic number 80 and atomic mass of 200.59. It is commonly known as quicksilver and was formerly named hydrargyrum. A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. It reacts as temperature increases by expanding to a predictable amount; this property made it useful in thermometers until they were phased out in favor of less toxic alternatives.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide.
Why is Mercury used in thermometers?
Mercury is used in thermometers because it has high coefficient of expansion. Hence, the slightest change in temperature is notable when it's used in a thermometer. Also, mercury has a freezing point of -38.83 °C and boiling point at 356.7 °C, this gives a very big range to measure temperatures for may general purposes, also it does not stick to (wet) the capillary wall of the thermometers, and its easy to obtain in its elemental form.
- It’s shiny. You can easily see the level of mercury inside a glass thermometer. Imagine using water or any other translucent liquid.
- It’s sensitive. Heating even on a small scale causes significant expansion in it’s volume which can be made clearly visible on a marked scale.
- It’s hard to boil. The boiling point of mercury is nearly 674.06 degrees Fahrenheit. So using it in college laboratory or hospitals really doesn’t convert the mercury to vapors.
- It’s non-sticky. The property of mercury to not stick on glass is a main reason to use it. Unlike water, droplets of mercury never stick on the glass walls of the thermometer.
- It is very reflective, so it's easy to see and to read accurately.
- It doesn't wet the glass, so you don't get inaccurate readings if the temperature is falling.
- It is a metal, so it's a good conductor of heat. This means that it reacts quickly to changes of temperature.
- It expands evenly with temperature, so a linear scale can be used with quite a high degree of accuracy.
- There's a large range of temperature for which it is a liquid. However, it freezes at - 39 Celsius, so Mercury cannot be used below this temperature. It boils at 365 Celsius, which is high enough for most purposes.
The Phenomenon Behind
Thermal Expansion and Contraction
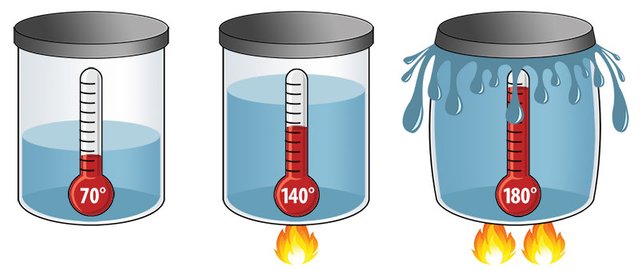
The volume of most materials increases as the temperature increases; scientists call this effect thermal expansion. The expansion occurs because the atoms in a material vibrate more strongly as you add heat energy to them; as the vibrations increase, the average distance between atoms also increases. For the same reason, materials contract as temperatures decrease. Each substance expands and contracts according to a characteristic quantity known as the coefficient of thermal expansion; this is a number that indicates the percentage of size change per degree Celsius. The expansion can be for an object’s volume, area or length; tables for each type of expansion list the substances and coefficients.
A. Mercury's Expansion
Mercury’s volume coefficient of expansion is 0.00018, so it expands by .018 percent in volume for every degree of temperature increase. To make the difference easier to see, a mercury thermometer contains a reservoir of the metal and a thin glass capillary into which it can expand. The mercury in the reservoir grows in volume as temperatures rise, but the walls of the reservoir constrain the mercury; it has nowhere to go but into the thin column, which converts the slight volume change into a more noticeable linear change.
B. Freezing Point
In addition to its expanding and contracting with temperature, liquid mercury freezes solid at -38.83 °C (-37.89 °F). Because of this, mercury thermometers have limited utility in very cold places where temperatures dip to below mercury’s freezing point. Alcohol thermometers, which freeze at much lower temperatures, are used instead.
C. Boiling Point
Liquid mercury responds to very high temperatures by boiling into a vapor; this occurs at 356.73 °C (674.11 °F). As with most liquids, reduced pressures lower mercury’s boiling point. To circumvent the limitations of mercury, high-temperature thermometers use thermocouples, infrared light sensors and other ways of measuring hot objects.
References
Websites:
- https://classroom.synonym.com/liquid-mercurys-reaction-temperature-32247.html
mercury reactance - https://en.wikipedia.org/wiki/Mercury_(element)
mercury deifinition
Author/Books:
- Yashkumar Sanariya, B.Tech Chemical Engineering, Sardar Vallabhbhai National Institute of Technology, Surat (2019)
- Yashkumar Sanariya, Chemical Engineer from NIT, Surat topics about Mercury in thermometer
- John Walker, BA Hons Natural Sciences, Retired Sience teacher, specialising in Physics: topics about 4. Mercury in thermometer
- Engineering Toolbox: Volumetric - Cubical - Expansion Coefficients of Some Common Liquids
- Georgia State University: Mercury
- Georgia State University: Thermal Expansion: How to Loosen a Jar Lid
Image Sources:
Introduction
Liquid Mercury
Element in Peiodic table
Vermilion
Mercury
Thermal Expansion
Mercury Expansion

Kindly give credit to the images that you are using. You can use this tool https://steemitcuration.appspot.com/imageformat.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Good day @bayanihan. Image sources' links were included in the end of the article. Nevertheless, thank you for the suggestion. I so appreciated that you noticed my posts. God bless and more power!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
G2Vape.com stands out as a leading destination for vaping enthusiasts. This website offers a comprehensive selection of e-liquids, vape devices, and accessories from top brands. Whether you're a seasoned vaper or just starting out, g2vape.com provides a user-friendly experience with detailed product descriptions and customer reviews. Their commitment to quality and customer service ensures a satisfying shopping experience. For anyone looking to explore the latest in vaping technology and products, G2Vape.com is a top choice.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit