A Result of Absorber
On this occasion I try to share a research result that I have performed and have presented before, a result of silica absorption with the influence of acid concentration and drying temperature in the preparation of absorbtion material from bagasse ash.
The results of silica gel preparation from bagasse at drying temperature at 10%, 15%, 20% and 25% HCl (Chloride Acid) concentration, 10%, 15%, 20% and 25% CH3COOH (Citric Acid) solution concentration, bagasse ash bag 80 gram. This research was conducted through a series of sodium silicate forming process which was followed by dry silica gel preparation.
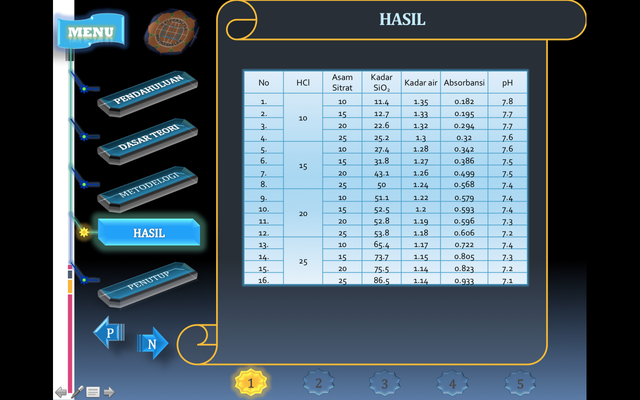
The process of preparing silica gel from rice husks in the first stage is the process of forming a solution of sodium silicate using NaOH as a catalyst. NaOH will react by binding to SiO2 contained in the bagasse ash and producing NaSiO2. This sodium silicate will be decomposed into SiO 2 or silica gel with the help of hydrochloric acid and citric acid of different concentrations.

Influence on SiO2 Levels
Silica gel (SiO2.xH2O) is a form of silica produced by the coagulation of a sodium silicate sol (NaSiO2). This gel-like solvent can be dehydrated so that it turns into a non-elastic glass-like solid or granular. This property makes silica gel utilized as an absorbent, catalyst and catalyst support.
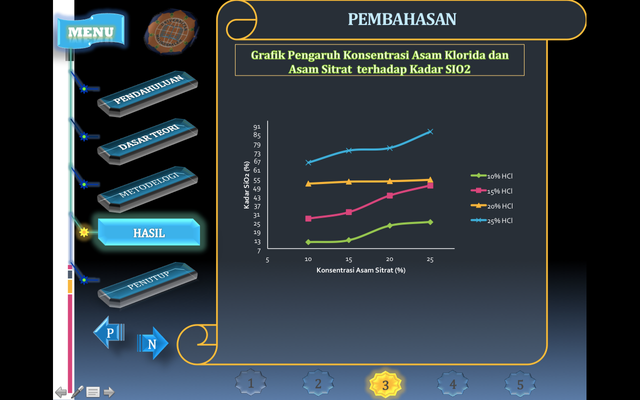
The purity of sodium silicate used is relatively low so that in general the effectiveness of the produced production is also relatively low. In this study, the concentration of hydrochloric acid and sistratic acid used were 10, 15, 20 and 25%. Synthesis of silica gel by using a solution of hydrochloric acid with a small concentration, will produce a little gel and gel formation takes a long time
Influence Of Water Content
Water content is the difference between the weight of the material before and after heating. Any material when placed in the open air moisture content will achieve balance with the humidity of the surrounding air. The principle of the drying oven method is that the water contained in a material will evaporate when the material is heated to 200 °C for 2 Hours.
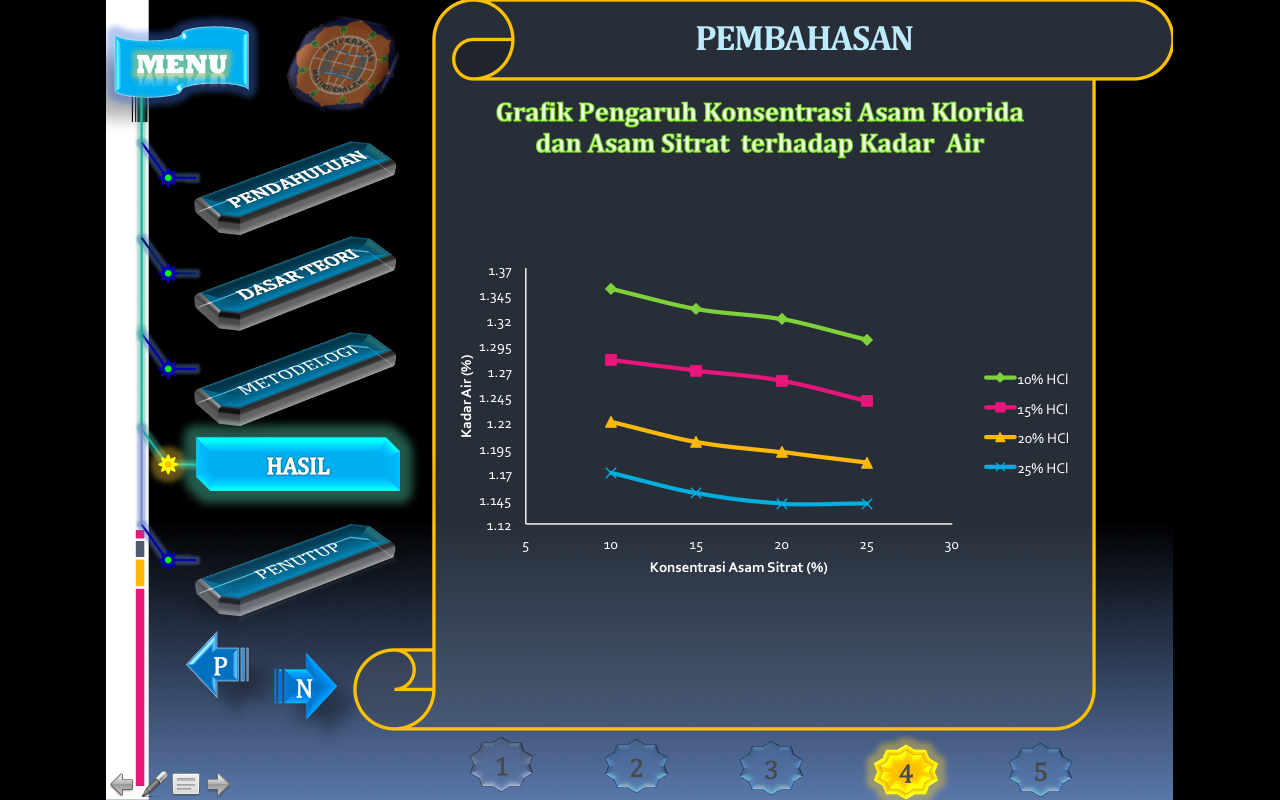
The water content in this study was defined as the amount of water released by silica gel due to heating at 200 °C for 2 hours.
Heating of silica gel at temperatures below 200 °C occurs the release of water that is weakly bonded to the surface of the silica gel referred to as physically bound water. Physically bound water can be evaporated at a relatively lower temperature than to evaporate water derived from the condensation of silanol groups into siloxane groups (Brinker, C. S. 1990).
The concentration of H + ions in an aqueous solution is generally very low but greatly determines the nature of the solution, especially the aqueous solution, in terms of the higher the concentration of an acid, the less the amount of water content.
Sample collected from sugar cane seller in Lhokseumawe, Aceh
Follow Me @jamhuery