Investigating the Effect of Concentration on Reaction Rate
Aim:
I want to investigate how concentration effects the reaction rate.
Equipment:
Conical Flask
Beaker
Measuring Cylinder
Stopwatch
Paper with a black cross "X"
0.1mol/L Sodium Thiosulfate
2.0mol/L Hydrochloric Acid
Method:
- Put the piece of paper with the X on the bench and put the conical flask on it.

- Measure 10mL of Sodium Thiosulfate and put it into the conical flask.

- Measure 40mL of Water and put it into the conical flask. Swirl to mix.
- Measure 5mL of HCl.
- Pour the HCl into the conical flask, start the timer and swirl the flask.
- Stop timing when the you can't see the cross on the paper.

- Wash out the flask thoroughly.
- Repeat experiment 20mL of Sodium Thiosulfate and 30mL of Water.
- Repeat experiment 30mL of Sodium Thiosulfate and 20mL of Water.
- Repeat experiment 40mL of Sodium Thiosulfate and 10mL of Water.
- Repeat experiment 50mL of Sodium Thiosulfate and no Water.
Results:
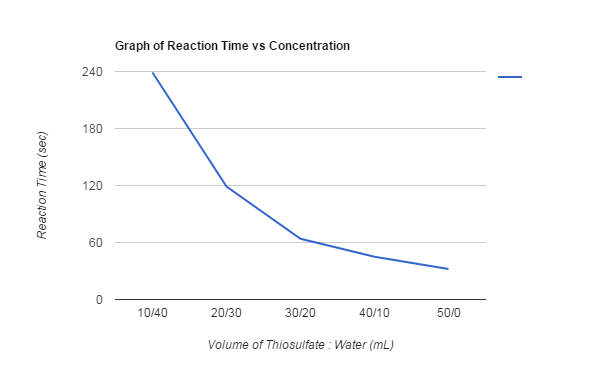
10mL Thiosulfate/40mL Water took 239sec for the X to disappear.
20mL Thiosulfate/30mL Water took 119sec for the X to disappear.
30mL Thiosulfate/20mL Water took 64sec for the X to disappear.
40mL Thiosulfate/10mL Water took 45sec for the X to disappear.
50mL Thiosulfate/0mL Water took 32sec for the X to disappear.
Analysis:
Conclusion:
A chemical reaction is when two reactant particles collide.
They must collide with enough force and in the correct orientation.
As I increased the concentration of the reactants, there were more reactant particles that were able to react. The more particles, the higher the chance of a successful collision.
This means the rate of reaction will increase.
Nice work! Now have you looked into how other factors such as temperature and solvent influence the reaction speed? - next steps!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thank you for the positive comment. I am slowly working my way through the factors of temperature, concentration, surface area and catalysts. I have not thought of the solvent influence. I look forward to trying out a few experiments where the solvent could influence the speed. I guess I would probably have to look at changing the polarity from polar to non-polar.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Kinetics/Reaction_Rates/Factors_That_Affect_Reaction_Rates#Solvent_Effects
See the solvent effect section!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit