Chloroplasts within plant cells. Source: Wikimedia.
Photosynthesis requires that this mechanism produces large amounts of chemical energy without losing the oxidative capacity needed to break water molecules. Now, a team of researchers headed by Masashi Hasegawa, from the University of Kobe (Japan), has made clear part of that mechanism, which is another step, not only for understanding the photosynthesis of green plants, but also in the development of artificial photosynthesis. Artificial photosynthesis would not only remove carbon dioxide from the atmosphere, it would also produce chemical energy at low cost, something that can be critical in combating climate change.
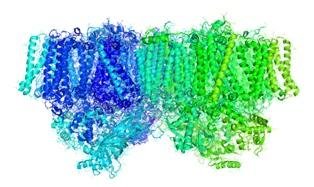
Structure of the photosystem II. Source: Wikimedia
During the water break phase of photosynthesis plants produce oxygen by converting solar energy into chemical energy, which gives the plant the energy it needs for its survival. This chemical reaction takes place in a protein complex found in chloroplasts, organelles present in the leaves, which is called photosystem II and which is rich in chlorophyll b.
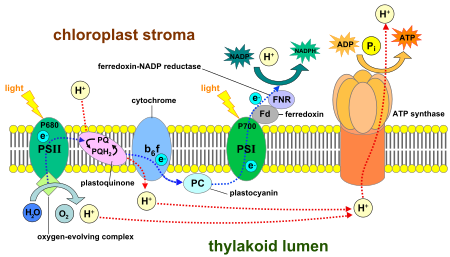
Luminous stage of photosynthesis, which occurs in the thylakoid membranes. Source: Wikimedia
In 2015, this same group of researchers was able to analyze the electronic interactions and the 3-dimensional localization of the electric charge separation that occurs just after the photo-reaction in the photosynthetic reaction center of a purple bacterium, but which does not produce the Oxidation potential needed to break a water molecule (photolysis of water). However, in photosystem II of the upper floors, the configuration of the initial charge separation is something that was not clear at all. In fact, it was a mystery how it could produce the effective breakdown of the water molecule while retaining a high oxidative capacity.
The methodology developed by the researchers has allowed them to carry out for the first time a 3D visual analysis of the configuration of the electric charge produced just after exposure to light. The precision obtained was less than ten millionth of a second between one image and the next.
Based on these images, researchers have been able to quantify the electronic interaction that takes place when the electronic orbitals of different photosystem molecules occupy the same space and, therefore, how easy the neutralization of charges can be. The overlap of orbitals has been found to be very limited by the insulating effect of the terminal vinyl groups. This makes possible that the great oxidative capacity of the positive charge of chlorophyll is maintained and can be used in the decomposition of water.
In this way we have a mechanism that is able to efficiently produce chemical energy without losing the oxidative capacity. The verification of this mechanism and the use of the developed methodology would facilitate access in a few years to truly clean and efficient energy sources that could be used for transport and industry, something, as we have said, critical to combat climate change.
References:
Masashi Hasegawa et al (2017) Regulated Electron Tunneling of Photoinduced Primary Charge-Separated State in the Photosystem II Reaction Center J. Phys. Chem. Lett., 8 (6), pp 1179–1184 doi: 10.1021/acs.jpclett.7b00044
https://commons.wikimedia.org/wiki/Commons:Licensing

Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You got a 12.42% upvote from @cabbage-dealer courtesy of @mofeta!
100% of earnings are paid out to delegators daily! Delegate now!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You just received a 14.29% lifting from @botox ! You can also earn by making delegation to @botox.
Tu viens de recevoir un lifting de 14.29% de la part de @botox ! Tu peux également être récompensé en faisant de la délegation à @botox.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You got a 1.37% upvote from @moneymatchgaming courtesy of @mofeta! Please consider upvoting this post to help support the MMG Competitive Gaming Community.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You got a 8.55% upvote from @proffit courtesy of @mofeta!
Send at least 0.01 SBD/STEEM to get upvote , Send 1 SBD/STEEM to get upvote + resteem
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You got a 10.81% upvote from @redlambo courtesy of @mofeta! Make sure to use tag #redlambo to be considered for the curation post!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You got upvoted from @adriatik bot! Thank you to you for using our service. We really hope this will hope to promote your quality content!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit