-- Experiment
1•compound (Naphthalene Date I There are three states of matter which may be inter-converted either by heating or cooling.
2•Melting point is a temperature at which the solid changes into Background liquid.
3•At this temperature solid and liquid state coexist.
4•It is a measure of th strength of intermolecular force Covalent compounds have low melti Information points but ionic compounds show higher points Crystalline compounds show fixed and sharp melting points.
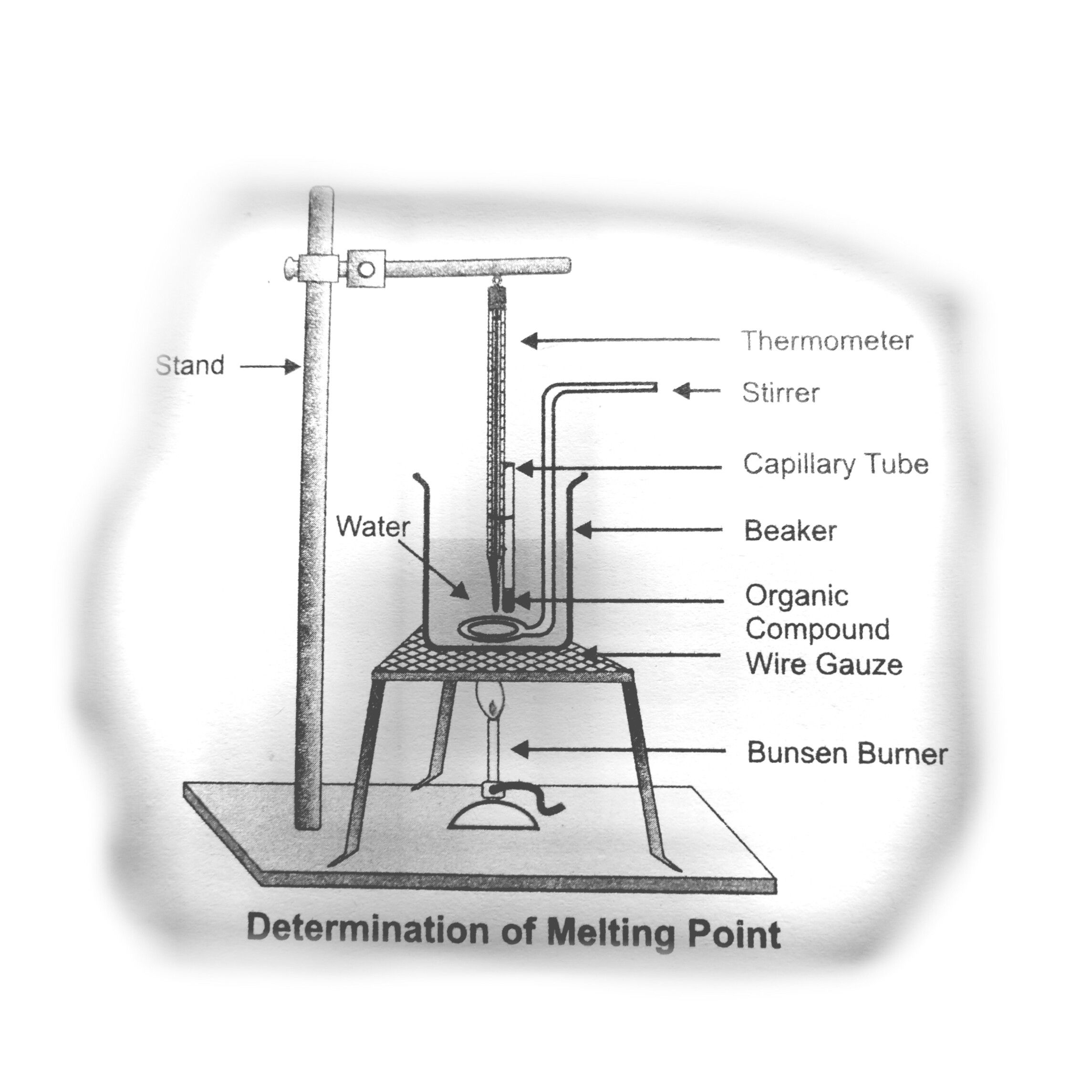

5•Thermometer, beaker (250 cm), throad, capillary tube, tripod stand, wire gauze. Materials Required stirrer, iron stand with clamp water and given organic compound (Naphthalene) ,
•Close one end of the capillary tube by heating it on the flame of Bunsen burner.
• Crush the organic compound (Naphthalene) to make its powder.
• Close one end of the capillary tube by heating it on the flame of Bunsen burner.
• Crush the organic compound (Naphthalene) to make its powder.
•Fill one centimetre length of the capillary tube with the onganic compound.
•Fill one centimetre length of the capillary tube with the onganic compound.
•Determination of the Point attach the capillary tube with thermometer with the help of a piece of thread.
•Keeping in view that the base of the capillary tube and the bulb of the thermometer must be at the same level.
•S Hang the thermometer with the clamp of iron stand using thread as shown in the figure.
• G Take 250 cm beaker and add approximately 100 cm ofwater in it.
• Place this beaker over the tripod stand with the wire gauze in between them.
• Now dip the thermometer along with the capillary tube into the beaker containing water as shown in the diagram The upper end of the capillary tube must remain 1 to 2cm above the water level.
• Bulb of thermometer must be completely immersed into water.
•Heat with continuous stirring Monte down the temperature at which the solid starts melting
•Repeat the experiment and note the melting point again.
•Calculate the average temperature and compare it with the standard melting point of the organic compound.
•••••••••Thanks for reading my {chapter no 2}>By.