Distillation can be seen as process that separates mixtures based on the differences in their boiling points or differences in volatilities. Fractional distillation is pretty much the same process but by the use of a frationating column.
A fractionating column helps to separate liquid mixtures with closely associated boiling points.
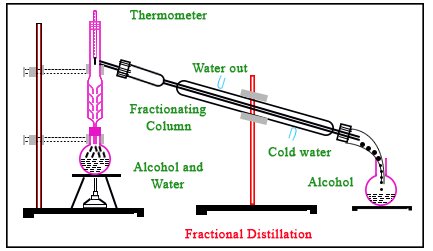 Source
Source
This process is usually employed in petroleum industries for separating petroleum constituents into the finished products like diesel, petrol, kerosene amongst others. Industrial distillation occurs in fractionating towers in the industry.
The raw crude is fed into the tower at a feed point and heat is applied at the bottom of the column. Constituents that have lower boiling points evaporate first before others and there are collecting points for each of these constituents.
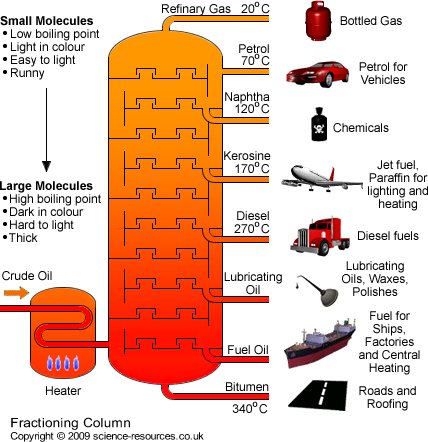
Source
Let me know in the comments if you have any questions on this topic so I can answer them. Cheers!!