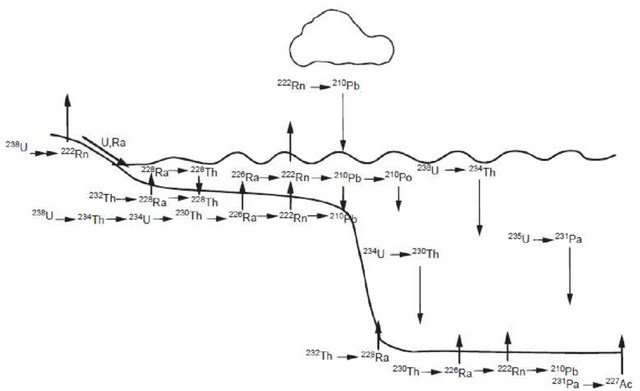
An unstable relationship.
Radioactivity is a natural phenomenon, presented by those isotopes in whose nuclei the relationship between protons and neutrons is unstable: either too large or too small. When the relationship is different from the graphic attached, the nuclei decompose, forming other nuclei more inside. For heavy nuclei, stability is achieved with a greater number of neutrons and the ratio between N and Z can reach up to 1, 5,6, deviating from the value 1 in the nucleus that is stable. Only some of these nuclei still exist on Earth in measurable quantities. It is very likely that there were others, but they have long since disintegrated and only traces remain undetectable.

The discoveries of Marie Curie.
In the description of radioactivity they were fundamental in the research conducted by Marie Curie. Both discovered elements that possess radiant activity or radioactivity, even greater than that of uranium: thorium, radium and polonium (the latter two discovered by the Curies). In all of them, the intensity of the emission was proportional to the mass fraction of the active element in the compound, as Bacquerel had sampled in the uranium compounds. From these results, they hypothesized that radioactivity is an atomic process that is not affected by physical soil (solid, molten, dissolved ...), nor by the presence of other non-radioactive elements. The fact that he called more the intention of this new radiation was of a continuous, persistent and spontaneous character, without requiring energy from an external agent for its maintenance and without apparently being exhausted.

Natural radioactive processes.
The idea of using plates to determine the penetrating power of the different radiations was due to the German physicist Philipp Lernard (1862-1947), who in 1895 showed that a stream of cathode rays (electrons) penetrates through sheets and sheets of different materials . This fact made us think that, although the sheets were very thin, the thickness of them contained many layers of atoms and therefore, the model of the atom could not be similar to a solid ball, but had to have an open structure. This idea was revealed shortly after knowing the different radioactive emissions.
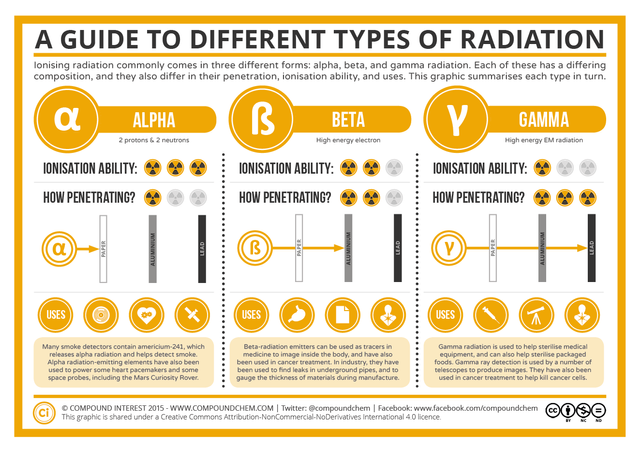
Types of radiation
From the beginning, experiments were carried out to check whether the radiation was unique or was composed of different components. Rutherford, in 1899, placed metallic sheets in the trajectory of the emission from a radioactive substance, and managed to characterize the different radiations. The first fraction called it alpha, which was absorbed by a small number of plates and did not suffer great deviation in a magnetic field; the second fraction had a penetration power 100 times higher and experienced a large deviation in the cited field; he designated it beta. Later Villard (1860-1934) detected the presence of a third more penetrating fraction, Gamma radiation. It did not affect the action of magnetic or electric fields, for this reason it was characterized as a high frequency electromagnetic radiation.
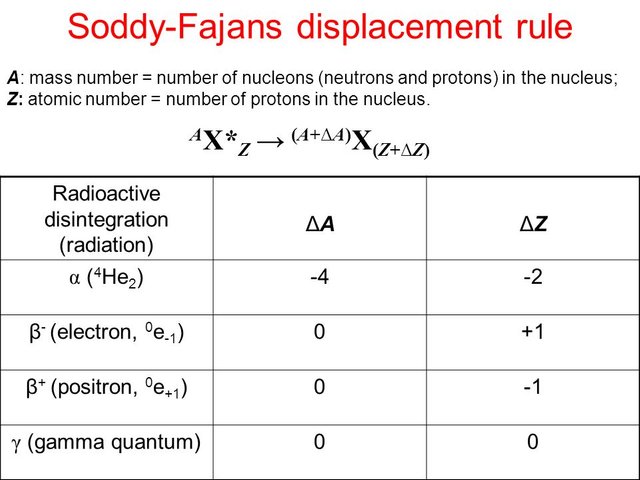
The laws of Fajans and Soddy.
The atoms emit radiations undergo modifications that transform them into other chemical elements that can be, in turn, Radioactive or not. The transformation follows a with as the laws of the displacement of radioactivity or laws Soody: First Law: When an atom undergoes an alpha decay, it loses 2 protons and two neutrons, so its mass number decreases by four units, while its number atomic decreases by 2 units. Second Law: When an atom experiences a Beta disintegration, an electron is created from the nucleus, for this same reason its mass number does not change and its atomic number increases by one unit. Since there are no electrons in the number, it is to be thought that a process of a neutron in a proton and an electron takes place inside the nucleus.

Source.
How long the radiation lasts.
The radioactive material is spent as the element is transmuted into other elements that may or may not be. The amount of radioactive material remaining in a given number depends on the time elapsed and the type of material. the number of existing reactive atoms is equal to: Useful radioactivity. Humanity uses to its advantage different characteristics of radioactivity to fine-tune techniques stops the application not only scientific. in this field it has been very important to obtain radioactive isotopes, which can be easily obtained through nuclear reactors when subjected to a neutron bombardment stable element samples. This produces short-lived radioisotopes that easily become stable isotopes. In all applications, it is essential to consider the half-life of the radioisotope, since it can be used appropriately.
This post has received a 10.61 % upvote from @boomerang.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit