Corrosion, Part 1

Corrosion
If you may have read my previous post, the application of corrosion may have come up quite often, and although a general idea of the destructive properties may have being given, I realized that it would be more informative for me to further explain and go deeper into the concept of corrosion, including its classifications, principles, rates as well as factors. By the end of this post, you will have a strong understanding of Corrosion.
Corrosion Concept
Corrosion is regarded as the deterioration of materials when it is encountered and reacts with the environments physical and chemical properties. Corrosion often mainly affects iron and steel products, although it can be noted to sometimes affect non-metallic objects such as ceramics and certain plastics. Corrosion occurs as a form of rust upon the material. Rusting is caused by the hydration (addition of water) of iron as well as contact between the iron and oxygen. It can therefore be stated, that since these requirements occur naturally, almost all areas of the environment are corrosive to a certain extent due to:
- Moisture in the air and soil due to watering systems such as hosing and irrigation.
- Air from the atmosphere that contains roughly 21 % Oxygen
- Other gases in the air that could promote corrosion, such as chlorine and hydrogen sulphide and even sea salt.
It was observed that inorganic matter is more corrosive than organic matter. This is because inorganic matter has more attributes that can initiate corrosion while organic matter does not. For example, sodium chloride, Sulphur and even hydrochloric acid are components found in the petroleum industry. The containment vessels of these fluids are more susceptible to corrosion due to these fluids being inorganic, while the containment vessels of oil would not have such a problem as most oils produced are organic oils. More severe results of corrosion are noted in the processing industries as high temperatures as well as pressure promote the rate of corrosion with the use of inorganic fluids. Corrosive environments are not only limited to liquids but also to gaseous and vapours as well as solids. A special branch of engineering referred to as Corrosion Engineering is dedicated to the control as well as prevention of corrosion damage upon a material in a safe and economical way.
Corrosion is a major problem that is affecting the world daily. Not only does it give a visual displeasing sight, it also accounts for a large expenditure in the citizens as well as the government’s budget. Corrosion affects and jeopardizes the following sectors:
The biggest factor of concern in the aforementioned list is safety. An example of safety endangerment due to corrosive affinities is the collapsing of the Berlin Congress Hall. This incident took place in 1980 in Germany. The hall was a gift from the United States of America to Germany due to the Berlin World Exhibition in 1957. The building stood for many years but due to been ill treated and not properly looked after, the steel components in the construction started to break due to stress corrosion cracking upon joints in construction. Another example is the failure of the landing gear equipment to engage during the descent of a F/A-18 aircraft. Due to the corrosion that occured upon the wing-fold and due stress corrosion cracking upon the landing gear connected to the wheels, the flight incurred almost $ 238 million worth of damages but amazingly had no casualties which is of more importance.
As can be understood, cost is a large factor to deal with due to corrosion. In the country of South Africa for example, almost 5 % of the Gross National Product is spent in the first quater of a year on Corrosion prevention as well as restoration due to corrosion. This type of cost incurred is referred to as a Direct Cost. A Direct cost is any expense that is related to the corroded material. There is also a branch of expenses referred to as Indirect Cost. These are expenses that relate to any metaphorical expense that costs you time and integrity rather than physical money.
Examples of direct costs include:
- Loss of affected material
- Cost of Repairs and maintenance
- A new design strategy
- Use of newer and more costlier equipment
- Transportation of new equipment and so on

Direct and Indirect Costs
Examples of indirect costs include:
- Loss of production time due to plantation shutdown
- Liability in the case of a casualty
- Loss of appearance in mobile cars and buildings
- Loss of peoples trust in the companies reliability and safety
The study of corrosion in industry can be extremely benefical to any aspect of the world. It will ensure product reliability as well as personal safety.
Corrosion Classification
Since corrosion can occur due to so many conditional requirements, it was easier to group classifications together to understand which branches need to placed on higher priority due to its extent of corrosion. The branches are as follows:
- High and Low Temperature Corrosion
- Direct Combinaton Corrosion
- Electrochemical Corrosion
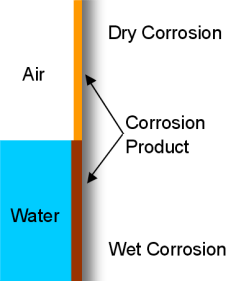
- Wet and Dry Corrosion
The top placed priority branch due to its large extent of corrosion world wide is Wet and Dry Corrosion. Wet Corrosion occurs only when there is a liquid phase available to be in contact with, such as an electrolyte in galvanic corrosion or even in aqueous solutons. Dry Corrosion occurs when no liquid phase is present or when the environmental temperature is above its Dew Point.
The addition of small amounts of certain elements, with the interaction of water, may have corrosive tendencies. If we look at chlorine, dry chlorine upon a metal or steel surface has non-corrosive abilities at all, but, with the addition of water, wet chlorine will become violently corrosive and tend to attack all available metal alloys.
Corrosive Principles
The process of corrosion can be stopped to a certain degree in everyday life. Treatment of metal is often used to slow down corrosion but this may cause additional costs to the company or individual, therefore, the choice of material used is important, in terms of corrosion behaviour. The following points are criteria to be aware of when purchasing materials:
- Materials Cost
- The materials Availability
- The Visual Appearance of the material
- Materials Strenght
- Method of materials fabrication
These 5 points are important when making decisions upon what material is optimal for a certain application. If it something around a house that needs to be chosen, the price range chosen be appropriate for its application , whereas, we do not expect the price of certain materials used in industrial construction to be as cheap as a household product. The point of reliability as well as cost should be thought upon together to find relatively good priced, reliable products. Knowledge of other factors including Thermodynamics, Electrochemistry, Metallurgy as well as Physical Chemistry will also assist in the purchasing of the optimal material, dependent on its uses.
Corrosion Rates
The rate of surface thinning or penetration into a metals surface is referred to as Corrosion Rate. It is used to calculate how fast or slow a metal loses weight due to corrosion thus helping scientists identify which metals have high corrosive resistance and which do not. If corrosion rates are not calculated correctly, equipment may get damaged as well as endangerment of lives, therefore it is very important too use simplified, accurate equations. Corrosion rate can be expressed in a number of units:

W = weight loss at the time of exposure (mg)
D = metals density (g/cm^3)
A = surface area exposed (in^2)
T = lenght of exposure in time (hrs)
If we have to use the above equation is SI units, the new equation becomes:
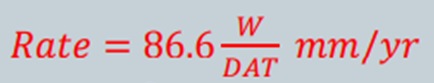
whereby A is now expressed as , A [=] cm^2
The amount of metal, W, can also be mathematically determined by use of Faradays Equation:
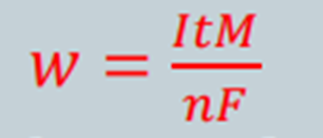
where,
W = mass of corroded metal (g)
I = current (A)
t = time (s)
M = molcular mass of the metal used (g/mol)
n = number of electrons lost/ its change in valency
F = Faradays Constant = 96 500 C/mol
An alternate method of calculating Corrosion Rate is:
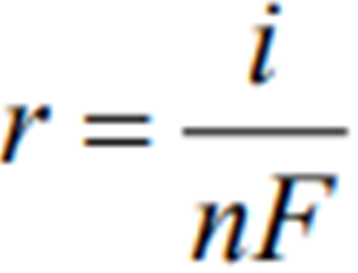
where,
r = rate (mol/m^2*s)
i = currrent density
n = number of electrons in valency change
F = Faradays Constant
Corrosion Factors
Corrosion can occur due to a number of reasons and they are as follows:
- The type of surrounding environment
- The environmental temperature
- Atmospheric pressure at the time
- The metals chemical constitution
- The metals surface condition
- Exposure time
- Structure of exposed metal and so on.
This brings us to the end of the post and I do hope that everyone reading this now has a better understanding of corrosion. On my next post I will go further into detail as to explaining the different types of corrosions that occur and how each of these can be prevented.
Images are linked to their sources in their description
The End
References:
[1]https://en.wikipedia.org/wiki/Corrosion
[2]www.electrochem.org/corrosion-science
[3]https://www.thebalance.com › Investing › Commodities › Metals
[4]frictioncalculator.com/corrosion-rate-formula
[5]https://www.emedicalprep.com/study-material/chemistry/.../factors-affect-corrosion/
[6]www.paintingforpainters.com/types-of-corrosion.html