- aluminum is one of the most interesting and abundant chemical elements. it represents about 8 percent of the mass of the Earth's crust; Unlike some other metals such as gold and silver, aluminum does not exist in a pure state in nature, It is always united with other components.
- Aluminum element in periodic table symbolized by Al. it is a used in the aircraft industry due to his lightness; and it's widely used in many products in our daily life like: kitchenware, furniture, toys, deodorants, indigestion medicines, parts in aircraft and missiles and the famous aluminum foil used in the kitchen for the frying of foods.......

Credit - in 1827 the aluminum was isolated for the first time in pure form by Wohler (German chemist).
- the very reductive nature of aluminum makes its preparation very difficult.
- currently aluminum remains a relatively expensive metal, which slows down its use, despite interesting properties.
- Physical and chemical properties:

Credit
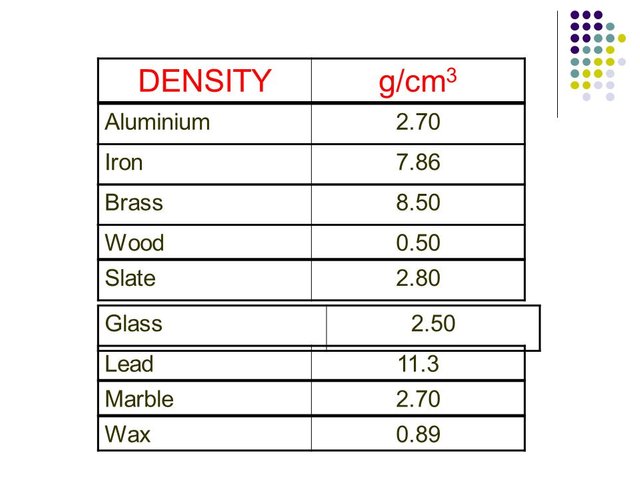
Density:
Credit
aluminum is the lightest of the common metals, (density = 2.7)Change the physical state:
the melting point of aluminum is 660 ° C. and its boiling point is 2056 ° C under normal pressure.
 Credit
CreditConductivity:
it is one of the best conductors of heat and electricity, this property is particularly interesting for the realization of electric power transmission cable.
Credit
Reflective power:
aluminum can be easily polished, it has an excellent reflective power of the light, it is used more and more for the manufacture of mirrors.

Creditmechanical properties:
aluminum is a soft metal, not very stubborn; its breaking load is about 50 to 60 N / mm2.Some of chemical properties:
- aluminum has a simple electronic structure
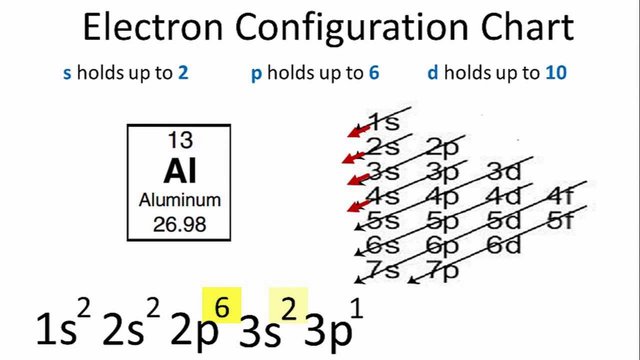
Credit - the atomic weight of aluminum is M = 27g.
- the atomic number of aluminum is Z = 13, which means that its nucleus contains 13 protons and 14 neutrons.
- the aluminum atom can easily be converted into an Al3 + ion by loss of the three peripheral electrons.
- aluminum is easily oxidized by the substances which remove electrons from it and which are themselves reduce, despite this, its corrosion is very low due to a phenomenon of self-protection.
Aluminum in nature:
a) Bauxite

Credit
bauxite is hydrated alumina which is written schematically Al2O3, H2O. it is the only aluminum ore on an industrial scale.
the very pure bauxite is white, but it is rare. most often, it is colored red by the presence of impurities, mainly iron oxides.
b) crystallized anhydrous alumina

Credit
it is the corundum, almost as hard as diamond, when impurities give it beautiful colors, it is a precise stone: ruby, sapphire .....
........................................................................................................................................................................................................
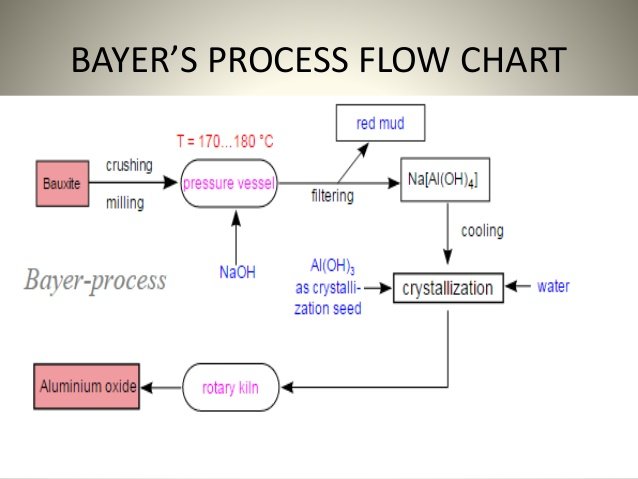
A- Preparation of pure alumina:
the Bayer process is based on the amphoteric properties of the hydroxide, there are several stages:
Credit
1- bauxite attack:
the ore is treated with soda in autoclaves, hydrated aluminum oxide is dissolved in the form of sodium aluminate:
Al2O3 + (2Na+) + (2OH-)======== 2AlO2- + (2Na+) + H2O
after decantation or filtration, the insoluble impurities are removed.
2- decomposition of sodium aluminate:
the filtered solution containing sodium aluminate is then left at a slow cooling; by addition of water, the basicity is reduced and the aluminum hydroxide precipitates according to the inverse reaction of its formation:
(Na+) + AlO2- + 2H2O ========== Al(OH)3 + (Na+) + OH-.
the aluminum hydroxide is separated from the solution by decantation and filtration.
3- Calcination of aluminum hydroxide:
calcination at high temperature (T = 1250 ° C) removes water and transforms the hydroxide to powdery oxide (white powder): 2Al(OH)3 =============== Al2O3 + 3 H2O.
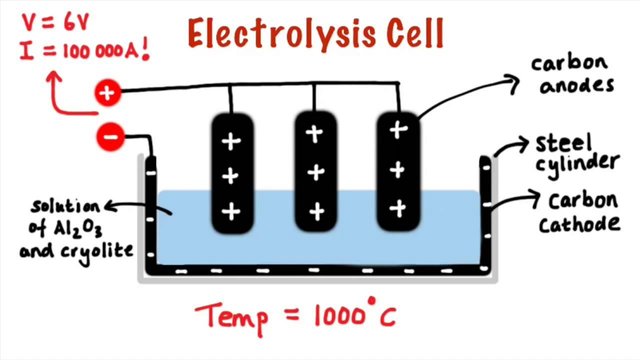
B- Preparation of aluminum:
Alumina is reduced to aluminum by electrolysis.
Credit
- aluminum is obtained at the cathode, carbon dioxide and carbon monoxide at the anode.
- the manufacture of one ton of aluminum requires a consumption of about 450 kg of coal.
- energy is important, we need an energy of the order of 15,000 Kwh per ton of aluminum.
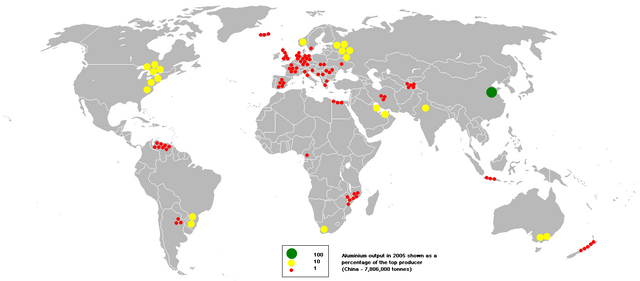
Production of aluminum throughout the world:
Credit
Can aluminum be recycled?

Credit
Aluminum is 100% recyclable, Recycling includes scrap smelting, a process that requires only 5 percent of the energy used to produce aluminum from crude.
- Recycled aluminum is called secondary aluminum, but it maintains the same physical properties as the original aluminum.
Aluminum and its alloys:
harder and tougher than pure metal or better at molding, alloys are used in many fields in life:
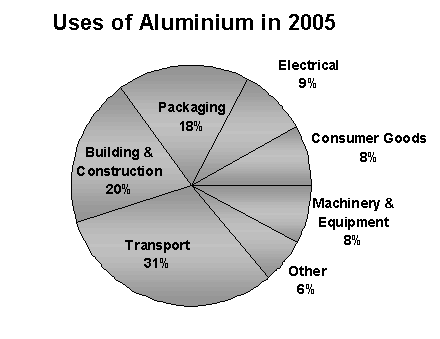
Credit
in the aerospace industry where the quality sought is above all lightness. alloys with good resistance to thermal variations have been developed for the construction of supersonic aircraft.
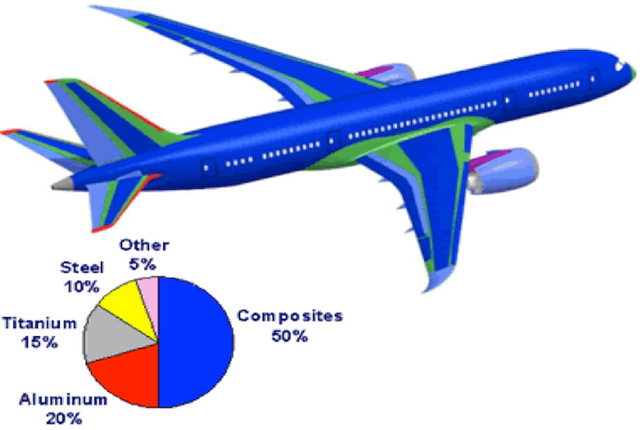
Creditin the automobile: molded parts in engines, decoration of bodies, road tanks ............

CreditFor building purposes such as for roofing materials, windows, doors, fronts and curtains

Credit
Manufacture of electrical equipment

CreditPackaging Applications

CreditPetroleum and chemical industry components
Rail transport

CreditShipping
Aluminum alloys are endurance against corrosion due to salt water and so you find applications in boats, ships, marine terminals, and other components.

CreditAluminum pneumatic batteries

CreditA large range of household appliance

CreditStreet lighting poles

CreditElectronics

CreditAluminum powder is used in paint, and fire industries such as solid rocket fuel.
CreditAluminum for deodorant industry

Credit
The Hidden Killer:
The element of aluminum is charged by people interested in the health of the human body as a dangerous element if it enters the human body. It causes a large number of health problems, the most important of which are anemia, Alzheimer's, osteoporosis, encephalitis, chronic fatigue, some cancers, Crohn's disease and some skin diseases. Studies have shown that aluminum can be found in a large number of medical and therapeutic preparations, some food, cosmetics, and even infant formula.so where is the truth?

CreditAluminum is a toxic element if the human body enters. The danger is that our bodies can not get rid of it. It accumulates in the cells of the body and causes complex and serious health problems.

CreditThe use of aluminum in some antiperspirants can increase the risk of breast cancer, Where research has confirmed high levels of aluminum in the nipple fluids of women with breast cancer.

CreditIn 1970, a study was published on Alzheimer's disease, specifically on finding large proportions of aluminum in the brains of people with Alzheimer's disease.
Aluminum is a reasonable price option and features an excellent heat connection, but it reacts with acidic foods and can cause various diseases in the long term.

CreditAluminum is among the main factors that reduce plant growth in acid soils.

CreditPrevention is better than treatment. It is better not to use aluminum foil in food wraps at low or high temperatures. Giving up such harmful habits will only cost us a crucial decision for the safety of our food and the preservation of our most precious health.
The water pollution of the 1990s caused an aluminum malfunction in Camelford, where a woman contracted arthritis, caused the death of river fish and many animals died on farms. A truck driver unloaded aluminum sulphate in the wrong tank and pumped water for drinking in the province.

Credit
References:
Initiation a la chimie moderne, ANDRE CROS; GILBERT ARRIBET
Chimie 1eC, G.Guinier and R.GUIMBAL
La géochimie recreative, G. Fresman
Aluminium, wikipedia
dangers of aluminium, al3oolom
الالمنيوم, المعرفة
Documentary, aluminium; aljazeera
- dear steemians, thank you for reading i hope you will like this post.
- If you like this post upvote and follow To be informed of upcoming posts.
- Accept my greetings and wishes for your health and wellness.
@Benainouna
Apart of the medicinal part I strongly support your fact collection about aluminium. For this part I would like to see more supporting, credible, primary literature. Otherwise nice job. :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you for giving your opinion for this post and thank you for your time, i will try to do better next time :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
that's amazing man .. thanks
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
you welcom, thank you :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Very informative, good job.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
nice write up
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Resteemed to over 12400 followers and 100% upvoted. Thank you for using my service!
Send 0.200 Steem or 0.200 Steem Dollar and the URL in the memo to use the bot.
Read here how the bot from Berlin works.
We are happy to be part of the APPICS bounty program. APPICS is a new social community based on Steem. The presale was sold in 26 minutes. The ICO will start soon. Read here more: https://steemit.com/steemit/@resteem.bot/what-is-appics
@resteem.bot
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
It never ceases to amaze me that something so toxic is found in many things we eat or that are injected into our bodies. I think people would be amazed to know where this is hiding and how damaging it is for people. Why the public has not had a problem with this boggles the mind.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
most of people don't know about the dangers of some elements, that's why people get ill more than before, the more we use chemical elements the more we will deal with health problems :(
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
So very true and until the public starts taking an active role in educating themselves we will continue to get sicker. Why people do not question things more is simply ridiculous, sheeple.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit