All of us hear about Chlorine, this abundant chemical element in nature, his most important derivative is "table salt" or sodium chloride (NaCl), The latter is necessary for many forms of life. for many years chlorine is used in many fields the best known is the field of detergents.

Picture source
This element (chlorine) of symbol Cl is something that is much important and useful and have many benefits for our lives, but we often forget about the dangers of using so much of materials having chlorine which can make many health problems , and those materials we are using everyday in our daily life so that's why all people must know about chlorine and know how to use it in safe way and protect ourselves Of its dangers. but before talking about benifits and dangers! we need first to know more about Chemical properties; Features and uses of this element......

Picture sourceHistorical:
The word chlorine comes from the Greek khlôros meaning "pale green". The chlorine was discovered in 1774 in the state of dichlor (Cl2) by the chemist Carl Wilhelm Scheele; It was in 1810 that Humphry Davy named it chlorine, emphasizing that it was in fact a distinct chemical element.where to find chlorine?
Chlorine is never found in the free state in nature, it is found combined with other elements such as sodium (Na) in the form of salt (NaCl) Extracted from seawater And rocks.

Picture sourcehow to produce chlorine ?! :
GAS EXTRACTION:
by electrolysis of a sodium chloride (NaCl) solution, or potassium chloride (KCl) solution, chlorine can be manufactured.
Chlorine-soda electrolysis is the main method of producing chlorine, There are three industrial methods for the extraction of chlorine by electrolysis of chloride solutions, all proceeding according to the following equations:
Cathode: 2 H+ (aq) + 2 e− → H2 (g)
Anode: 2 Cl− (aq) → Cl2 (g) + 2 e−
Overall process: 2 NaCl (or KCl) + 2 H2O → Cl2 + H2 + 2 NaOH (or KOH).
1- Mercury cell electrolysis.
2- Diaphragm cell electrolysis.
3- Membrane cell electrolysis.
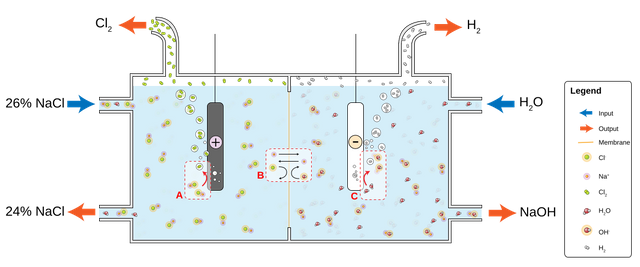
Picture source
- Chlorine and economy :

Picture source
The chlorine industry plays a leading role in global industrial and economic development. Today, more than 50% of turnover, more than 30% of investments and around 25% of jobs in the global chemical industry are based on chlorine, soda and derivatives activities.
___________________________________________________________________
Chemical Properties of chlorine:
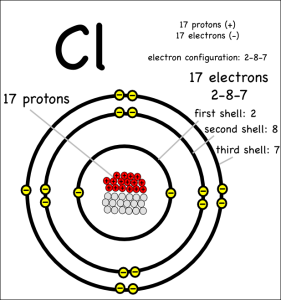
Picture sourceThis element is a part of the halogen series
Atomic Symbol: Cl
Atomic Weight: 35.453 g.mol -1
Atomic Number: 17
Molecular Weight of Cl2: 70.906
Electronegativity according to Pauling: 3
Density: 3.21*10 -3 g.cm -3 at 20 °C
Melting point: -101 °C
Boiling point: -34.6 °C
Electronic shell: [Ne] 3s23p5
Energy of first ionisation: 1255.7 kJ.mol -1
Standard potential: - 1.36 V
- Sources of chlorine in food :

Picture source
Chlorine is available in nature in the form of sodium or potassium chloride in several foods such as: fruits, vegetables, food salt, milk products, grains, meat, and birds.
- What is the importance of Chlorine in human body?

Picture source
Chloride is present in small amounts in cells, but abundant in extracellular fluids: interstitial fluid located between cells and blood. It is an important factor in balancing the quantities of water inside and outside the cells. It is involved in the regulation of the pH (level of acidity) blood. In the stomach, it is used to manufacture hydrochloric acid, constituting gastric juice (which participates in the digestion of food).
it's also help perform the functions of both the nervous system and the muscles and Maintains the water balance and distribution of fluid in the body.
- Chlorine and drinking water:

Picture source
-Using chlorine for Sterilization of drinking water is global procedure that helps cleansing Biological pollutants, and such biological pollutants have killed thousands of people over decades.
Despite all the great services that chlorine has given to mankind But the risks are not negligible, It requires immediate action and radical solutions to rid humanity of the dangers of chlorinated water; Because drinking chlorinated water causes diseases of the colon and Bladder.
Many studies have shown the ability of chlorine in water to interact with a number of organic compounds present in water. This reaction results in a large group of dangerous and carcinogenic chemical compounds, most notably THMs and chloroform.
The danger of Bathing and swiming !!!

Picture source
_Showering and bathing in chlorinated water is not less risky than drinking it, It is found that chlorinated water is dangerous to those who use it for bathing, as hot water helps to lighten the pores of the skin and thus allow the chlorine in the water or that is present in the shower atmosphere through the skin to all parts of the body internal.
_The process of showering with chlorinated hot water and inside a small enclosed bath leads to the saturation of the surrounding atmosphere with chlorinated water vapor and carcinogens. Thus, such dangerous substances will be inhaled either through the respiratory system or through the skin.
Briefly Chlorinated water can cause very serious diseases such as:
َAsthma, sensitivity, sinus problems, sye recognition, cough, shest pain, scute infections in the airways, she fluid collects in the lungs, heart disease, repeated cases of miscarriage..........
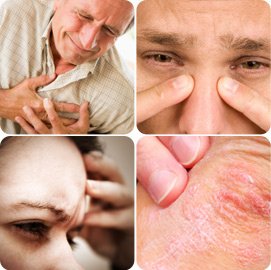
Picture source
. Bathing or swimming in Chlorinated water also causes skin irritation and hair loss:

Picture sourceChlorine as a weapon in war:

Picture source
. Chlorine itself is not flammable, but it can react to the formation of explosives with other chemicals such as turpentine and ammonia oil.
. Chlorine was used during the First World War as a chemical weapon that causes suffocation.
- Using of chlorine and industry:
. Production of paper products.
. Production of disinfectants.
. Production of dyes.
. Production of insecticide.
. Oil products.
. Production of Plastics
. Medical Industries.
..............
References:
http://www.techno-science.net/glossaire-definition/Chlore.html
https://fr.wikipedia.org/wiki/Chlore
https://www.futurelearn.com/courses/exploring-our-oceans/0/steps/730
https://www.chlorineinstitute.org/stewardship/chlorine/chemical-properties/
https://fr.wikipedia.org/wiki/%C3%89lectrolyse_chlore-soude
https://hal.inria.fr/file/index/docid/252458/filename/ajp-jp4199404C116.pdf
https://en.wikipedia.org/wiki/Chlorine_production
https://en.wikipedia.org/wiki/Chlorine_production#Gas_extraction
https://www.lenntech.com/periodic/elements/cl.htm
http://www.doctissimo.fr/nutrition/oligo-elements/chlore-chlorure#sources-alimentaires-de-chlorure
http://theweirdlife.com/microbe-mondays-chlorinated-water/
http://al3loom.com/?p=20122
http://mawdoo3.com/%D9%85%D8%A7_%D9%87%D9%88_%D8%A7%D9%84%D9%83%D9%84%D9%88%D8%B1#.D9.81.D9.88.D8.A7.D8.A6.D8.AF_.D8.A7.D9.84.D9.83.D9.84.D9.88.D8.B1_.D9.84.D8.AC.D8.B3.D9.85_.D8.A7.D9.84.D8.A5.D9.86.D8.B3.D8.A7.D9.86
http://www.egyres.com/articles/%D9%85%D8%A7%D8%B0%D8%A7-%D8%AA%D8%B9%D8%B1%D9%81-%D8%B9%D9%86-%D8%BA%D8%A7%D8%B2-%D8%A7%D9%84%D9%83%D9%84%D9%88%D8%B1%D8%9F
- dear steemians, thank you for reading i hope you will like this post.
- If you like this post upvote and follow To be informed of upcoming posts.
- Accept my greetings and wishes for your health and wellness
@Benainouna
I think d dangers is more than d advantage, it's kinda sad many people dont know about it's effect.. that's y I'm resteeming this.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank u very much for giving us important information about the chlorine .
I didn't know about it before after reading your post I have come to know about it.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you for finding time to ready this post, and i'm glad you like it :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you for your time, yes i hope all people know about this dangers :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Interesting and quite relevant. Thanks for this post
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
you welcom :) ,thank you for reading and i'm glad that you find this post interresting :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Could be better... Try using marksdown format to hide your links.. [write anything here] ( paste link here).
There should be no space between the brackets
– @pangoli
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you, that's what i was looking for :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Well that truly was a fascinating read and you'v given me an explanation to why I'm bald at thirty.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you for your time, so i hope other people will be carefull to not get blad at thirty lol
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
So that's one of the reason why I'm getting bald. Fuck clorine! Give me back my hair!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
lol be careful next times
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This is lovely. I have learnt alot from this. Weldone
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
i'm glad you like it, thank you for your time
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I think chlorine as a weapon of war would be a war crime and could prove to be biologically devastating... People could die getting their lungs deteriorated by inhaling it...
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
it's already a war crime, but who respect laws in war!!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
i think that the percentage of advantgae is big
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you for your time, like any other chemical element we need to know how to use it in a safe way to to benefit from advantages
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You got a 0.52% upvote from @postpromoter courtesy of @benainouna! Want to promote your posts too? Check out the Steem Bot Tracker website for more info. If you would like to support development of @postpromoter and the bot tracker please vote for @yabapmatt for witness!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
wow! great and well researchedarticle @benainouna. I always think about taking a daily shower as getting a "dose" of chlorine!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
thank you :) i'm glad that you find this post useful @troutmusic
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit