In my previous publication I spoke about how Lorentz and Zeeman obtained their Nobel Prize, this was thanks to the work done on the electronic theory of light, but before this discovery I explained to them in summary form everything that happened before this discovery, with the Faraday and Gauss experiments and later Maxwell's equations where he unifies the electric and magnetic field.
But on this occasion I will go deeper into the work of Lorentz and Zeeman. If you wish to know more about the history of the discoveries made before the work on "The influence of magnetism on radiation phenomena" and the subsequent appearance of the "Zeeman effect", I invite you to read my previous installment and thus you will be able to easily digest this new publication.

Next I will approach the Lorentz theory in a very simple way (no equations) so that you can understand the nature of how electromagnetic radiation originates, as well as explain Zeeman's experiment and its effect, something that could complement the Lorentz theory. All this through the approach of modern physics or commonly known as quantum physics.
The principle of Lorentz's theory was that "all the electrons were the ones that caused the emission of light", according to "this is very true", but it could not be approached that easily, because in order to study the behavior of this phenomenon it is necessary primarily to apply Maxwell's equations and perform a quantum study to be able to understand this phenomenon. Maxwell said: " that the electrically charged particles have an acceleration and therefore emit an electromagnetic radiation", if their electric charge is very big or it is increasing, the more radiation it can emit (it increases its frequency, it decreases its wavelength).
We all know that electrons are the source of electromagnetic waves, but in reality they are not the only ones because any particle with an electrical charge can be an electromagnetic wave. A clear example of this is if we have particular for example the α and we shoot them towards a metallic plate, it is logical that the metal slows down these particles and the result would be the emission of an electromagnetic radiation. When we think about this we could imagine the X-rays, right?.
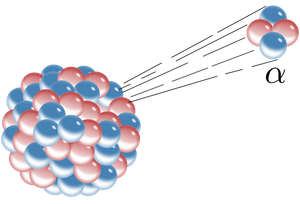
Alpha particle (α). CC BY 3.0
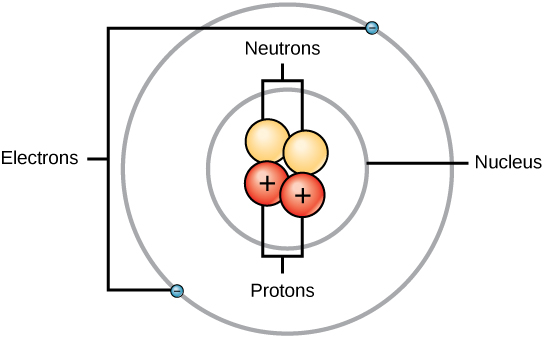
Electrons are the source of most electromagnetic waves in our space, something Lorentz had claimed 100%. But then maybe we can ask ourselves and couldn't another particle be the source that could emit electromagnetic waves?..ummmmm very good question for those times no?... Could protons be?...
The answer is simple: we have two reasons that can answer this concern, one is that electrons are particles that can move easily, ie can jump from one level to another with an injection of voltage, instead the protons are more static, are more attracted to the nucleus. Since electrons can be released and move easily as for example in metals, when moving from one side to another, from layer to layer or orbit, then they suffer a kind of acceleration due to the application of voltage and because of this they emit electromagnetic radiation. Because of this we have endless applications today, such as antennas. We know that what determines the energy of the wave emitted is the acceleration of the charge, and this despite the fact that both the electron and the proton have equal magnitude, so if we subject both particles with equal magnitude of force, the electron will always accelerate much more than the proton because its mass is much smaller.
In spite of all this, during the years following the work on the influence of magnetism on radiation phenomena , the appearance of the Zeeman effect, and outside of Maxwell's equations, we have been able to study, experiment and discover different sources of electromagnetic waves that are not associated with accelerated charges, all of which are supported by fabulous theories.
Now out of Maxwell's theory and his famous equations and after the electromagnetic theory of light formulated by Lorentz and the Zeeman effect has been studied, experimented and discovered different sources of electromagnetic waves to which does not correspond accelerated loads, all this is governed under the study of the famous theories such as relativity and quantum. To talk about this is a little complex and for that I would need like 10 publications hehehe lie :P, in the same way I intend to complement a little the previous post and continue talking about the works of Lorentz and Zeeman. Let's continue...
Now, by fusing two elements that are helium and hydrogen this produces that the mass can be converted into energy and causes a release of electromagnetic radiation, this is how life is maintained on earth, thanks to this type of reaction, the energy that allows the hydrostatic balance of the sun. Then we can see that in the nuclear reactions the waves that emit these processes have greater energy than those produced by the particles of electrons in the atom in hot bodies.
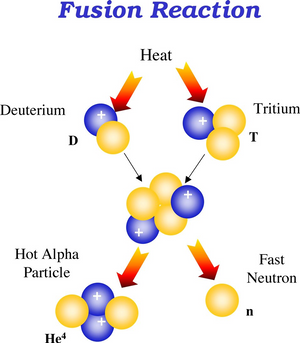
Fusion reaction. Public domain
None of this (nuclear reactions) was known at that time, although electronic models were a great advance at that time, there were still things that needed to be explained. Lorentz did an excellent job explaining the origin of electromagnetic radiation that existed in space, obviously with the help of Maxwell's equations, but in the air there were many questions. Something that became clearer little by little and above all with the Zeeman effect. So if we know perfectly well that the electromagnetic radiation was generated by the electrons, because the elements emit certain light of very specific colors? or because they could not emit light of any wavelength? or also that it was what caused these lines to be fixed in the spectrum?.
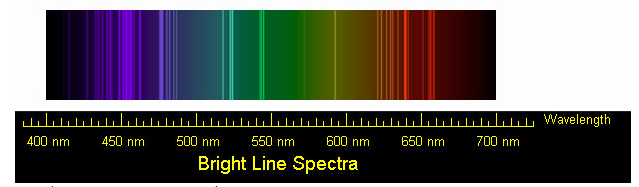
Sodium spectra. Public domain Wikipedia
A clear example of this is when Zeeman used Lorentz's theory and began conducting experiments to confirm what he was saying. One of these is when he took an element like sodium and heated it, the answer to this was that it emits radiation with different wavelengths, something that previously Lorentz could not explain in his theory, so most of these wavelengths emitted two orange lines which I call D1 and D2 each with 589 nanometers, but between those wavelengths no light is emitted.
Although the questions were still in the air Lorentz tried to explain what happens to sodium when it is subjected to a magnetic field and why those two lines were divided into three and said: that it was due to the movement that existed between the orbit of the electron, the atom and the magnetic field, the particle will always have its same energy, more or less, but the question was why the electron could only have one of those three energies?... can exist or form different spins at different degrees and if other lines are added between the spin axis and the field no others can be formed?...
As proposed by Zeeman sodium cannot split each line and then be three, on the contrary it should provide a varied group of lines between the narrowest and the longest wavelength, because the electron moving in any plane corresponding to the field generated, said this could stay with the same energy with which it started its orbit or gain more certain... then the initial lines and the split lines are related.
why only a few discrete values, and not just any values...?
But a little more was needed to be able to explain this behavior, because according to then matter should not exist right? because the same particles (electrons) that are in the atom should move towards the nucleus emitting continuous radiation.
But both Lorentz and Zeeman, the energy in question does not seem to be emitted or absorbed continuously, but rather at certain wavelengths. So what happens to the electron, does it gain energy?, does it lose energy?, or does it gain a little more?, or does it lose a little more?, what actually happens?... Let's get this straight...
Have you heard of standing waves? Of course I have... so here this type of radiation comes into play and of course having some basic knowledge of modern physics we can explain it as the electron inside the atom can possess certain energy and it behaves like a standing wave because it is inside or around the atom without being able to move. As a result of the radiation it emits or absorbs it belongs to a whole value of energy of the electron, that is not just any value.
So this answers the question of why the electron could only have those three energy options or that if different spins can be formed at different degrees and if other lines are added between the spin axis and the field no others can be formed? Since the electron when bound to an atom can only have a certain number of energy or a certain value, by this I mean that its orientations must be totally determined with respect to the magnetic field. Then Zeeman could observe that between the maximum and minimum energies of the lines there was only a small gap where more energy could be introduced. In conclusion it was three lines or more and not just one.
Interesting, isn't it?... Now let's explain a bit how the result of the Lorentzian theory applied to atoms today..
First of all we know that electrons have separate energy, this depends on the energy level in which it is located. Generally the electrons must be located in the lower levels, that is to say where they are freer, so that as they can get closer to the atomic nucleus it is ideal. Although we know that for example in conductive metals there may be a possibility that some of these levels are empty because the electron has moved to another level because it was excited, therefore it acquires more energy and can go to a level occupying another place where it can acquire even more energy than the previous one.
It is easy to understand because basically the place that is empty is quickly occupied by the electron due to the energy that it is injected into it, after occupying that place the surplus energy must be emitted in the form of a photon of the corresponding wavelength. Otherwise, when the electron is outside the atom, another electron must occupy its place, that is to say, it moves to a lower level, also emitting a photon of wavelength.
Finally we can say that the wavelength of an electromagnetic radiation depends on the difference of energy between the levels. If they are very close the photon will have little energy and in this case it would be the infrared radiation, if they are far away, the photon has a lot of energy and they will be the famous X-rays, and so on all the electromagnetic radiation spectra.
A simple publication easy to digest free of complex equations that by means of which those people who are not familiar with this type of subjects manage to understand with exactitude. It is the idea of this post, that many can read it without the need to know much about physics, just basic knowledge that you learned at school.
I hope you enjoyed this content...
Greetings...
Content support
source of cover images:
Publish through our official app and you will get an extra vote of 5% https://www.steemstem.io/
 Video credits @gtg
Video credits @gtg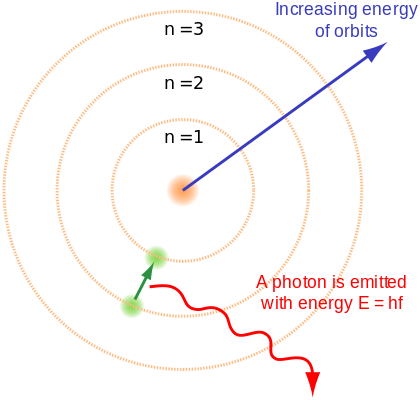

@tipu curate
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Upvoted 👌 (Mana: 10/15 - need recharge?)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks @ritch and @tipu
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Very interesting, thank you
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit