Hello dear friends of Steemit, I would like to talk to you about some material, revolutionary and with amazing chemical and atomic properties, this material has come to give a resounding change to the way of seeing things and understand them, and who could say, that all part of the carbon that is its main component, let's read a bit to understand it better.

Carbon is one of the most important chemical elements in nature. It is found in all living beings and, depending on how its atoms are distributed, it can form substances with different characteristics.
Graphene is obtained from carbon. This material arises when very small carbon particles are grouped very dense in very thin two-dimensional sheets (they are the size of an atom), and in hexagonal cells. To give you an idea, its structure is similar to that resulting from drawing a honeycomb on a folio. Why on a sheet? Because it is a flat, two-dimensional surface, like graphene.
Here we have a view of graphene under the microscope.
Graphene is obtained from a substance abundant in nature, graphite. This is part of our daily life since it is used to make a wide variety of objects, from the pencils mine to some bricks.
Although graphene is known since the 1930s, it was abandoned as too unstable. It was not until many years later, in 2004, when scientists of Russian origin Novoselov and Geim managed to isolate it at room temperature. This discovery was not trivial because thanks to him they obtained the Nobel Prize in 2010.
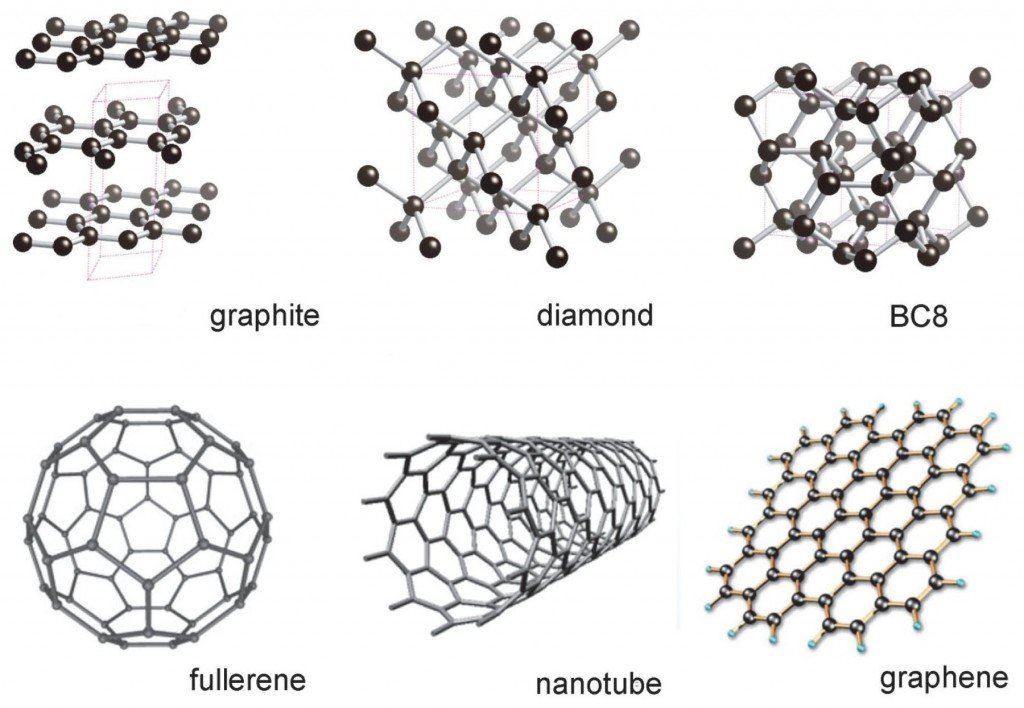
Graphene is the basic elementary unit in 2D to build all the graphitic materials of the other dimensions. For example, it can be arched in zero-dimensional structures (0D), as in the case of fullerenes, it can be rolled into 1D structures, giving rise to carbon nanotubes and, finally, it can be stacked successively giving rise to three-dimensional graphite (3D)
According to the IUPAC (International Union of Pure and Applied Chemistry), the term graphene must be used when talking about "reactions, structural relationships or other properties of individual layers" of carbon. Bearing this in mind, it is not correct to describe graphene as "graphite layers" (graphite implies 3 dimensions while graphene implies carbon unions in two directions), "carbon sheets" and similar concepts. Thus graphene can be defined as an infinitely alternating polycyclic aromatic hydrocarbon of rings of six carbon atoms, that is, it would be a flat molecule composed of carbon atoms that form a pattern of hexagonal rings.
Featured properties and features of graphene
Graphene is a substance with very interesting characteristics, some amazing. These properties together with the abundance of carbon in nature have made graphene gain the adjective "material of the future". Some of the most outstanding features of graphene are:
• High thermal conductivity.
• High electrical conductivity.
• High elasticity (deformable).
• High hardness (resistance to scratching).
• High resistance. Graphene is about 200 times stronger than steel, similar to the strength of the diamond, but it is much lighter.
• It is more flexible than carbon fiber but just as light.
• Ionizing radiation does not affect you.
• It has a low Joule effect (heating when driving electrons).
• For the same task, graphene consumes less electricity than silicon.
• It is capable of generating electricity by exposure to sunlight.
• Graphene is a practically transparent material.
• It is very dense and does not allow helium to pass in gaseous form, however, if it allows water to pass, which, enclosed in a graphene container, shows an evaporation rate similar to that shown in an open container.
Other characteristics still under discussion are the capacity for self-cooling described by researchers at the University of Illinois or their ability to self-repair. If a layer of graphene loses some carbon atoms for any reason, the atoms near the gap left approach and close the gap, this capacity for self-repair could increase the longevity of materials made with graphene, although in a limited way.
The properties of graphene make it an ideal material for multiple applications in technology, especially in electronics in the manufacture of integrated circuits. It is assumed that the characteristics of graphene can make it possible to build processors much faster than current ones.
This speed has already been put into practice in the manufacture of field effect transistors built with graphene. These transistors also take advantage of the high mobility of carriers with a low noise level that graphene presents.
Bulletproof vests and body protection
One of the most interesting properties of graphene is its extraordinary hardness. If we combine the moldability that it shows, we have an incredibly resistant material as a result.
One of the future uses that have been raised for graphene is destined for the arms industry. In particular, its usefulness would be aimed at shielding and protection.
Therefore, helmets, bulletproof vests, and many more accessories could be manufactured. It could, in fact, be a determining material for the future of police forces and armies.
Even motorists could have protective helmets much more resistant to possible bumps or abrasions.
Smartphones more powerful, flexible, resistant and durable
The world of technology would be one of the great beneficiaries of the standardization of graphene as a material to incorporate in products such as smartphones or tablets.
It would be the definitive step to make disappear the concept of mobile to which we are accustomed, passing to a higher stage.
Graphene could be a radical change, with completely flexible screens, even folding, which would mean be able to fold the smartphone like a handkerchief, to put it in your pocket, without the properties of the phone and the material with which If they were done, they would be affected.
But not only would be more resistant and flexible screens. Graphene would also improve the speed of the processes. And it is that the circuits that integrate the electronic terminals could be more efficient with graphene.
This would result in the creation and incorporation of faster and more powerful processors and even of a smaller size.
The battery of the smartphone could also be improved thanks to graphene. The lithium would go into the background to make way for graphene.
The properties of graphene and its treatment focused on batteries could make the life of a smartphone last up to a week without having to charge it.
In addition, they would only have to be a quarter of an hour connected to the current for their full charge.

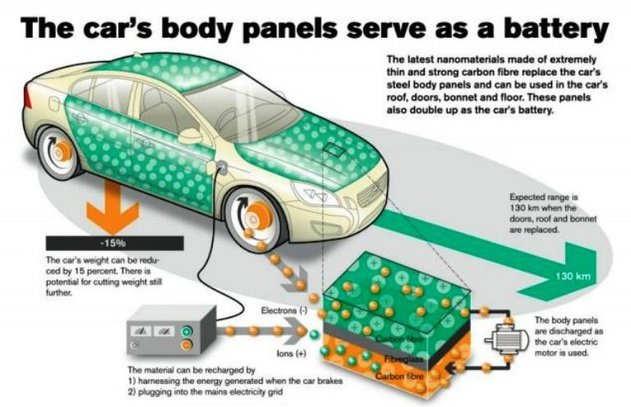
Cars resistant to shocks and abrasions
The extraordinary strength and hardness of graphene, coupled with the flexibility of it seems perfect to start creating or mass-producing cars immune to shock.
Finally, accident-proof vehicles could be created. This would result in a direct decline in road mortality.
But graphene would not only fit into the chassis of the vehicle. It would also find space for its application in the heart of the vehicle. Specifically, on the battery and the engine.
As in smartphones, graphene batteries could also be found in cars, making them more durable and reducing their charging time.
Nowadays, the automobile companies are starting to extend the hybrid engines although at the moment they do not reach the market share that the diesel or gasoline engines have.
This trend could be reversed thanks to graphene, which could be included in hybrid engines to make these cars, vehicles better prepared for this type of engine.
In addition, scientific advances are already demonstrating that graphene could be used to extract hydrogen from the atmosphere. If combined with fuel cells, this technology could make it possible for electric cars to feed on small amounts of hydrogen in the air.
Some other potential applications for graphene
• Distillation of ethanol at room temperature for fuel and human consumption.
• Ultra-sensitive gas detectors.
• Optical Modulators.
• Graphene transistors.
• Faster and more efficient integrated circuits.
• Transparent electrodes.
• Electrochromic devices.
• Solar cells.
• Desalination
• Antibacterial applications.
The main current problem in the application of graphene is its production. At the moment the investigations in the production of the graphene go by the exfoliation of the graphite transferring sheets of graphene from the graphite and by epitaxial growth.
Apart from the problem of the production of graphene in reasonable quantities and costs for its use, there are other arguments to ensure that graphene will not replace silicon in electronic devices or is the technological panacea with which it often occurs. For example, graphene does not present resistivity (electrical resistance) with which silicon does. This lack of electrical resistance means that graphene cannot stop driving electricity, which can be a great inconvenience.
Famous scientists in the field of technology, such as the physicist Walt De Heer, support the use of graphene as a new material with which you can do things that silicon can not do but in no way be a substitute.
For more information, visit this pages:
https://computerhoy.com/listas/life/7-usos-mas-curiosos-del-grafeno-que-llegaremos-ver-23937
http://tinomenosesmas.blogspot.com/2011/06/que-es-el-grafeno.html
https://www.uam.es/ss/Satellite/es/1242655278903/1242653870327/notcientifica/notCientific/Elsa_Prada_nos_explica_que_es_el_grafeno,_el_material_del_futuro.htm