These posts are not for foraging. They are intended for entertainment and intellectual satisfaction only. These posts are not a field guide nor comprehensive in any way - their accuracy is not assured in any way. Do not eat wild mushrooms unless you are a professional, have substantial professional assistance or have a wealth of personal experience with a specific species. Do not make any foraging decisions based on these posts. To do so could be dangerous or life threatening.
These Posts Contains No Information Regarding Edibility Or Toxicity

This is a roughly 20 to 40x magnification macro photo of the slightly exposed gills of a fresh, young Agaricus bisporus mushroom
Today's post is groundbreaking for me on several fronts, and introduces two major new sources of amazing imagery.
First, let's talk about the photo above, and the photos below. I purchased a 40x jewelers loupe or hand lens in order to better observe macro characteristics of mushrooms - i.e. cap texture, gill structure, etc etc.
The loupe arrived a few days ago and I was super satisfied with its quality. Then I thought to myself, how cool would it be to be able to take photos of what I'm seeing through this lens?! But, that would take an expensive camera and lens combo...unless...
I put my phone camera right up to the loupe lens and lo and behold!
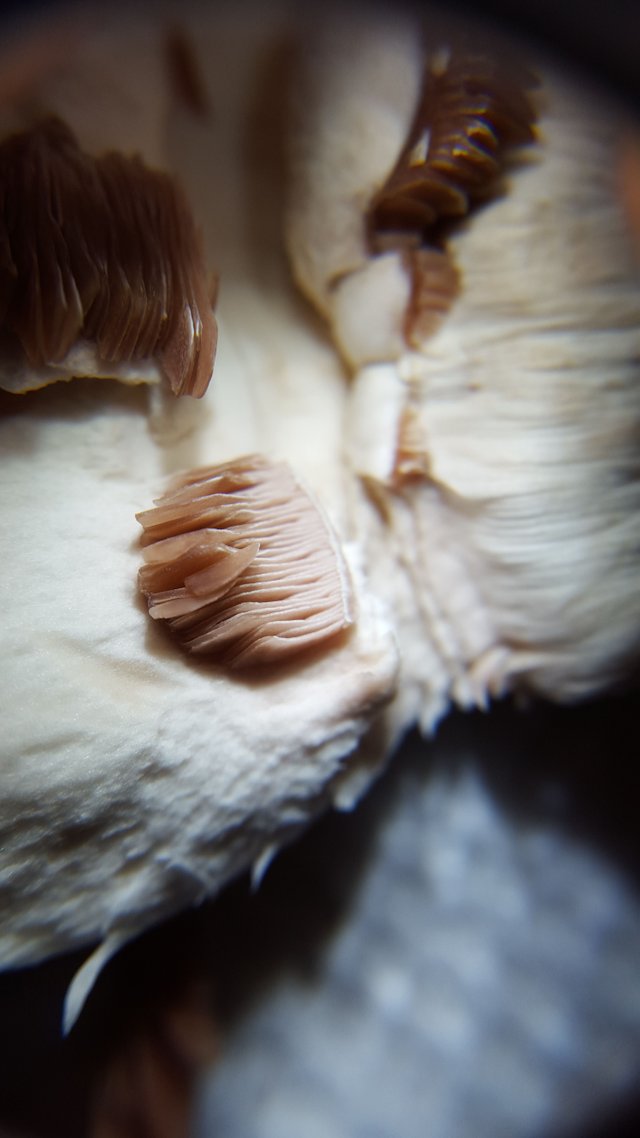
The results were awesome beyond my wildest expectations.
I never thought this would work! I guess it isn't so surprising that it does, but it's such a major enhancement of my phone's macro photographic potential for just $20. In the first photo the gills have not yet been disturbed and you are looking at them under a lip of white cap. This second photo better highlights the waxiness of the young gills.
But take a look at this cross section in order to get a sense of how thick they are

These gills are not yet entirely mature, which is why they are still quite fleshy and not dark brown.
But as time passes - just a couple more days really - the gills will stretch, become longer and grow darker and thinner as the spores mature and begin to be expelled.
Which leads us to the next, even more important addition to my mycological arsenal - some legit microscopic work.
After cleaning and calibrating my scope, I was ready to take it for a test run. What better way to accomplish that then to examine a mushroom whose every detail is incredibly well known.
Before I began, it was important to identify what it was I was looking for in this test. I still have no colored stains, and my microscope has no oil immersion lens and only goes up to roughly 400x. Nonetheless, I felt there were at least a few things I could search for and likely find which were specific to A.bisporus mushrooms:
- The rough spore shape and fairly precise size - possibly the color; and
- The shape and size of the basidia
There are other micrscopic details you could look for, but if I could tease out these two things I would have the primary microscopically relevant information for the species.
Glossary: Basidia are the microscopic structures that collect and eject mature spores from the spore producing surface of Basidiomycota - the name for the groupong of the bulk of the mushrooms you know and love.
With my goals set, the first step was learning how best to mount my specimen

This is a 40x magnification of my first finished mount.
For a very detailed breakdown of what Kuo refers to as the "Roman aqueduct mount" check out his page on the topic, along with his other pages on microscope use, which is also source 2 below.
The general idea is straight forward. Take your mushroom and with a razor blade slice as super thin a slice as you can down through the cap and then perpendicularly through the gills. The goal is to have a super thin, perfect cross section of the mushroom which would allow you to examine every surface, plus the paper thin interior.
This all sounds well and good, but turns out achieving this is not that straight forward. Personally, my mount was not that successful. Plus I treated it with KOH, but had no colored stain to apply, so everything just got fairly translucent and mushed together.
I ended up taking a gill specific route and tried something different.

I just broke off an entire gill, making sure to remember which side faced downward on the mushroom, and then put it all onto a slide.
This had mixed, but ultimately successful results. The gill is still quite thick. Ideally, the mount would be even thinner than this gill is wide. As a result, it was difficult to see much beyond the edges.
But the edges were all I needed. First, I found a sea of basidioles.
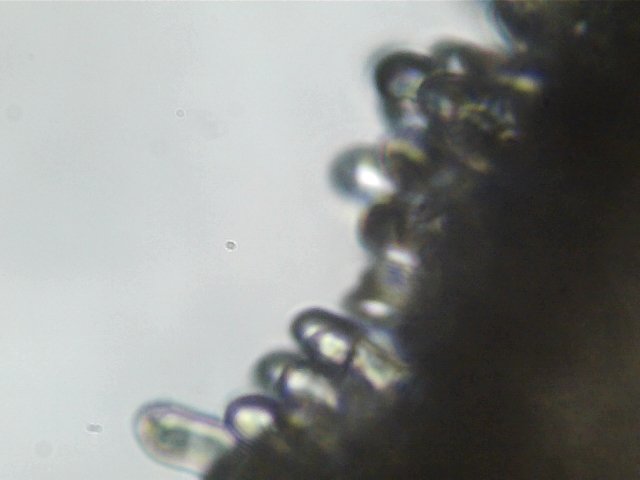
Those clear, featureless tubes are basidioles
A basidiole is a basidia which is either not yet totally mature or failed to develop entirely. It makes sense that I would see so many basidioles on this gill because the mushroom is not yet mature.
In fact, the mushroom was so immature, that I decided to return later. Which I did, about 24 hours later. By this time the mushroom had left a very pretty dark brown spore print and the gills were thinner and darker.
I mounted a sample and dived back in
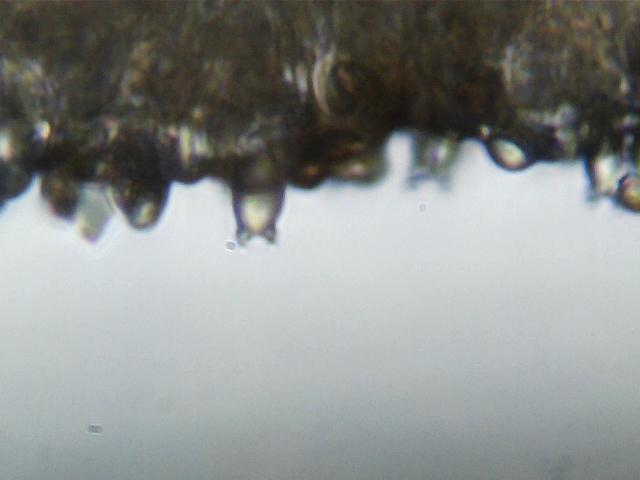
I soon found a series of strange looking, cartoonish structures - like a globular torso with two tiny arms - this is a basidia!
However, this basidia has expended its spore load already. That is why the arms are empty - where it used to hold two spores, those spores have been ejected, and now it holds nothing. This basidia, and the other blurry basidias also in this photos, have served their purposes.
Remember this mushroom is called A.bisporus? That "bi" is a reference to one thing only - the two pronged, two spore holding basidia.** By finding two pronged basidia, even spent ones, I had confirmed the primarily important microscopic trait of this species!
But I was not satisfied - I got myself more and more comfortable manipulating the slide and eventually got the clear, unequivocal picture I desired.

There it is - totally unequivocal in its bi-sporus-ness.
This is a loaded, ready to fire A.bisporus basidia, magnified to about 400x. You can clearly see the globular body, the two attaching prongs, and the two spores loaded at the end, ready to be fired off.
The "bi" portion of the bisporus name is more than just a microscopically visual difference. Whereas many gilled mushrooms have 4 spores on 4 prongs -the prongs are also called sterigmata - A.bisporus has only two.
However, this reduction in numbers is deceptive, as the two spores loaded onto the basidia here are both fertilized and ready to grow into a new mycelium. This is contrary to many other basidia bearing mushrooms, where the ingredients for procreation are split between different sets of spores, each of which must then find a "mate" before reproduction can occur.
This, right here, is the heart of the mushroom. When we say that mushrooms, the large fleshy mushrooms we are all familiar with, are simply reproductive implements, this microscopic structure is what we mean. It is the basidia in most of the Basidiomycota mushrooms that collect and then release the mature spores, which then go about trying to reproduce. All over the gills of every gilled mushroom, there are millions and millions of these basidia loaded up with tens of millions of spores, ready to shoot them off into space in the hopes of reproduction.
As I became more facile with scanning the slide, I was able to get a small sliver of the scale of the basidia operations happening on this infinitesimal piece of gill. Take a look:
Row upon row of loaded basidia, ready to launch their spores.
If you've ever wondered why mushrooms are such a successful organism, look no further than that youtube video. And remember that this is just the tiniest sliver, of the tiniest gill, on a single mushroom with hundreds of similar gills. No wonder mushrooms, and their spores, are everywhere.
Having successfully pictured the unique basidia of A.bisporus, all that was left was a sample of the measurements of its spores.

These mature spores were brown and their shape was... well...not quite circular.
I say that because its funny how many ways various mycologists describe spore shapes. Kuo says elliptical. First-nature says oviod to subglobose. These are all basically synonyms. "Egg-shaped" would be roughly accurate, hence oviod. Subglobose just means not quite globose, which means like a globe. Elliptical basically means oviod, although it would also count as subglobose. Anyway, they are roughly egg shaped.
However, my microscope cannot be entirely trusted regarding exact color or shape. But, hopefully, size is a different story. I took a sampling of 6 of the mature spores and found that all of them fit within the ranges set by multiple sources - 4-8.5 x 4-6.5 µ.
Having taken a number of measurements of a known mycological quantity and found those measurements to be accurate and within the known range of that mushroom, I can now go about my mycological, microscopic business with a fair degree of confidence in the value and certainty of my measurements. As you can see, the ranges we are usually operating within make the .03 µ margin of error immaterial.
These last couple of posts have broken new ground for me in a couple of big ways. It's too bad that these advances have come so late in the season, but I am hopeful I will be able to put these new mediums to good use in the coming weeks. Regardless, I want to thank those who take the time to read and support these posts - and the good folks at @steemstem and @curie for their amazing and substantial support. More than any post so far, I really learned a great deal in terms of practical skill building this last week, and but for the motivation Steemit and this community provides, I wouldn't have learned anything new at all. Many thanks and many more explorations to come.
THIS POST IS NOT INTENDED FOR FORAGING PURPOSES AND TO USE IT FOR THOSE PURPOSES WOULD BE DANGEROUS. DO NOT HUNT WILD MUSHROOMS WITHOUT RELYING ON A COMBINATION OF PROFESSIONAL FIELD GUIDES, IN PERSON PROFESSIONAL GUIDANCE, OR IN PERSON GUIDANCE BY SOMEONE TRUSTWORTHY WHO HAS COPIOUS LOCAL, SPECIALIZED MUSHROOM HUNTING EXPERIENCE. FAILURE TO DO SO CAN RESULT IN GRIEVOUS PERSONAL HARM OR DEATH.
Photos Are My Own - Microscopic photos were taken using an AMScope M150B entry level microscope. If you use microscopes, I'm quite sure you've never heard of this model - but its cheap and available on Amazon. The camera lens is also AMScope, MD35 - by far their crappiest microscope camera. But, as we will confirm on Thursday, still capable of material, relevant, and in some cases, dispositive, data collection. Lastly it should be noted that the precise magnifications are not easily deduced using the camera - but based on relative spore sizes compared to known microscopic photos from Kuo and other sources, I estimate 40, 100 and 400x.
Information Sources - Reference 2 and 3 are not super relevant to this post, which was mostly calibration based on reference 1. But 2 and 3 are excellent resources and absolutely integral to the next post on Thursday.
[1]Super helpful video from AMscope on calibration - but all the equicpment is much higher quality than what I'm using
[2]Kuo, M. (2006, February). Using a microscope to study mushrooms. Retrieved from the MushroomExpert.Com Web site
[3]First Nature's excellent page on mycological microscopy introductions
[4]Me on Agaricus Bisporus, with relevant external sources cited
What an excellent post! All of the information was interesting and the walk through of your process was very interesting to follow.
To be honest it was this photo that pulled me in. The image is both specific and abstract at the same time. I realized right away that it was some kind of macro of a mushroom, but at the same time it looked like a fine dessert, with some delicious vanilla ice cream on top. The extreme angle of the light also makes for a very dramatic look.
Cheers,
@kellyjanderson
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks for the kind words! I am so excited about the photographic potential of this hand lens - hopefully this is just the first of a diverse series of photos
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Who would have thought our store bought white button mushrooms were such a complex organic machine. Amazing pics especially considering you are using a $20 jeweler's loop.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
The closer you look, the more complexity you seem to uncover in even the most ubiquitous things - there is a fractal element to all this "life" stuff.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Some really great microscopy shots in this post dber! Nice work.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks man! I really want to get better at it. I'm going to be doing more posts during the winter on known mushrooms that I can source in advance - hopefully by spring I'll be able to search for details I don't expect in advance.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Sounds great!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Beautiful and informative! This kind of quality content is exactly why I joined Steemit.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Excellent information! Will be following along.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
i resteem
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Nice.. thank you for sharing:)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit