Medicines from plants: The first recorded use of plants for medicinal purposes was found on Egyptian
papyrus dated about 1500 BC. All over the world, from tribal medicine in Africa
to folk remedies in Britain, many thousands of plants have been used to cure
all sorts of ailments. Often, this knowledge has been handed down by word of
mouth, and there is now increasing concern the much of it will be lost for ever
as cultures change, tribes disperse and modern medicines of the industrialised
world dominate.
The bark of the cinchona tree was used for centuries by the people of Peru to treat malaria, and we now know that it contains the anti-malarial drug quinine. The Chinese use herbal medicines extensively: one of their herbal cures for asthma has been shown to contain ephedrine, which is used in modern medicine to enlarge the air passages of the lungs. Even aspirin has its origins in willow bark.

Interest in tribal and folk remedies has been reawakened since the 1960s. Chemists investigate thousands of plants each year to see if they contain biologically active chemicals that might be developed into medicinal drugs. There is real hope that newly documented remedies will lead to drugs that treat cancer, heart disease and HIV/AIDS.
INTRODUCTION
Many organic chemicals come from crude oil, many come from plants and animals and others are made synthetically. Chemists use separation techniques to isolate these substances, followed by analytical techniques to find out their chemical structure. The methods chemists use to identify the formulae of these organic compounds and determine their structures are the subject of this post.
Pharmaceutical libraries and stage synthesis
Although you may think of a library as a collection of books, to a pharmaceutical company it is a huge collection of compounds that may be useful as drugs. Only a few years ago most of the compounds in these libraries were naturally occurring compounds made by plants, animals or microorganisms. Chemists would make these complex compounds one at a time, and each would then be screened for any pharmaceutical activity. This was a slow and expensive process. Typically, a medicinal chemist might synthesise a hundred compounds in a year.
In the past few years there has been a revolution in building these libraries. Thousands of likely drug molecules can be built up in stages in a matter of hours using computerized syringes. Basic building blocks of chemicals are reacted at each stage; they combine and the resulting molecules become larger and larger.
Building up molecules in stages
Suppose the reactant molecules that a chemist wishes to combine are called A, B and C. At stage 1, the A, B and C molecules are placed in separate reaction vessels and shaken with polymer beads, often polystyrene. These beads are tiny and one gram will contain approximately one million. The A, B, or C molecules covalently bond to the polymer beads. This makes it easier to wash and separate the growing molecules at the end of each stage. The reactant molecules, attached to their polymer beads, are then split equally into three reaction vessels, so that each one contains a third of each reactant.
At stage 2, more of A, B or C is added to each of the three reaction vessels giving nine possible product molecules, all attached to polymer beads:
A-A, A-B, A-C, B-A, B-B, B-C, C-A, C-B, C-C
The polymer beads in each of the three reaction vessels are washed to remove any unreacted reagent. They are again split into three equal portions and mixed together in another three reaction vessels.
At stage 3, A, B or C is again added to give 27 different product molecules. Each is still attached to its polystyrene bead. The beads are washed and the mix and split is repeated a fourth time.
At stage 4, A, B or C is added. There are now 81 different product molecules.
Large-scale screening
By the sixth stage there are 46 656 product molecules. It would be no use producing this number of molecules if it took years to screen each one. Drugs are described as fitting into the receptor sites of molecules. These molecules are usually enzymes. To screen the thousands of molecules produced from combinatorial chemistry rapidly for potential drug activity, the molecules are split from their polymer beads and reacted with enzymes. Only those that affect enzymes by fitting into the receptor sites are developed further.

WORKING OUT THE FORMULA OF A COMPOUND
I am going to show how we can work out the formulae of completely unknown compounds, such as those that might be present in a medicinal herb. First, we need to understand what percentage compositions and empirical formulae mean.
PERCENTAGE COMPOSITIONS AND EMPIRICAL FORMULAE
One very useful piece of information about any compound is its percentage composition. This means the percentage by mass of each element in the compound. If you have just discovered what you think is a new compound, finding the percentage composition is one of the first investigations you would carry out. You can either decompose a known mass of the compound into elements, or burn it in oxygen and weigh the products formed, such as carbon dioxide and water (called combustion analysis). The masses of any other elements present in the compound are found by other means.
In the combustion analysis for carbon and hydrogen. A few milligrams of the compound being analysed are completely combusted. The carbon and hydrogen in the compound form CO2 and H2O, respectively. The increased masses of the two absorbent materials are measured to give the masses of CO2 and H2O produced. Given that the mass of the original sample is known, the percentage composition can be calculated. Once the percentage composition is known, the empirical formula of a compound can be calculated.
The empirical formula is the simplest whole-number ratio of the number of atoms of each element in a compound. For example, benzene has a molecular formula of C6H6 but its empirical formula is CH.
EXAMPLE 1
Combustion analysis shows that the compound that gives cinnamon its characteristic aroma has a percentage composition of 81.8 per cent carbon, 6.1 percent hydrogen and 12.1 per cent oxygen. Calculate its empirical formula. (Ar: C = 12.0, H = 1.0, O = 16.0)
SOLUTION
The simplest way to deal with this problem is to imagine that you have 100.0 g of the compound so that the masses of the elements are easy to work out as shown below. Remember that the amount in moles is the mass in grams divided by the mass of one mole in grams.
| Carbon | Hydrogen | Oxygen | |
| Mass in grams: | 81.8 | 6.1 | 12.1 |
| Amount in moles: | 81.8/12.0 = 6.82 | 6.1/1.0 = 6.1 | 12.1/16.0 = 0.756 |
| Simplest ratio: (divide by the smallest number) | 6.82/0.756 = 9.02 | 6.1/0.756 = 8.1 | 0.756/0.756 = 1.00 |
| Simplest whole-number ratio: | 9 | 8 | 1 |
So its empirical formula is C9H8O
Another way of calculating the empirical formula from combustion analysis data is to use the actual masses of the different compounds produced. As shown in the next example below.
EXAMPLE 2
0.100 g of a sugar, known to contain only carbon, hydrogen and oxygen, is completely combusted to give 0.147 g CO2 and 0.0600 g H2O. Calculate its empirical formula.
First, we need to calculate the masses of C, H and O in the compound.
There are 12.0 g of C in 44.0 g CO2 (Remember: 1 mol CO2 = 12.0 + (16.0 × 2) g)
Therefore, the mass of C in 0.147 g = 0.147 × 12.0/44.0 = 0.0401 g
There are 2.0 g H in 18.0 g H2O (1 mol H2O = (1.0 x 2) + 16.0 g)
Therefore, the mass of H in 0.0600 g = 0.0600 × 2.0/18.0 = 0.0067 g
Since we now know the masses of C and H in 0.100 g of sugar, the rest of the mass must result from O:
0.0401 + 0.0067 = 0.0468 g
Therefore, mass of O in 0.100 g = 0.100 - 0.0468 = 0.053 g
We can proceed now as we did in the previous example:
| Carbon | Hydrogen | Oxygen | |
| Mass in grams: | 0.0401 | 0.0067 | 0.053 |
| Amount in moles: | 0.0401/12.0 = 0.00334 | 0.0067/1.0 = 0.0067 | 0.053/16.0 = 0.0033 |
| Simplest ratio: (divide by the smallest number) | 0.00334/0.0033 = 1.0 | 0.0067/0.0033 = 2.0 | 0.0033/0.0033 = 1.00 |
| Simplest whole-number ratio: | 1 | 2 | 1 |
So its empirical formula is CH2O
FINDING THE MOLECULAR FORMULA
The empirical formula tells you the simplest ratio of different atoms in a compound. It does not tell you the actual number of atoms of each element in a molecule. To find the molecular formula, you need to know the relative molecular mass, M, of a compound, which is:
Mr = mass of 1 molecule of the compound/one-twelfth mass of one atom of carbon-12
The molecular formula may be the same as the empirical formula, or it may be a multiple of it. In the example above, the empirical formula of the substance found in the vinegar is CH2O. So we have:
Relative mass of CH2O = 12 + (2 × 1) + 16 = 30
However, Mr (CH2O) = 60, so because 30 × 2 = 60, the empirical formula must be multiplied by 2 to find the molecular formula:
(CH2O) × 2 = C2H4O2
If the empirical formula of sugar is CH2O, and in this case, Mr = 180. This is 30 × 6, so the molecular formula is:
(CH2O) × 6 = C6H12O6
So, when you analyse a compound, you need to know its relative molecular mass. This is where the mass spectrometer comes in.
MASS SPECTROMETRY
Mass spectrometry is the most accurate method of determining relative atomic and molecular masses, but it has many other applications. The mass spectrometer is used by geologists to date rocks, by anaesthetists to analyse compounds in a patient’s breath, by pharmaceutical companies to determine the structure of novel compounds and by the oil industry to work out where samples of crude oil originated. A mass spectrometer has even been taken to Mars to analyse rocks and dust on the planet’s surface and gases in its atmosphere. Clearly, the potential of mass spectrometry is enormous.
The mass spectrometer was developed in 1919 by the eminent English physicist Francis Aston, from apparatus used by J.J. Thomson (the discoverer of the electron). In 1922, Aston received a Nobel prize for his work in developing the mass spectrometer.

Replica of J.J. Thomson's third mass spectrometer. Jeff Dahl, CC BY-SA 3.0
Aston used his mass spectrometer to show that neon gas was composed of isotopes. Neon atoms were ionised, separated, and then made to hit a photographic plate. Two lines were produced, one much darker than the other, corresponding to relative isotopic masses of 20 and 22. By measuring the relative darkness of the two lines, Aston found that neon-20 atoms were ten times more abundant than neon-22.
From this information, he worked out the average atomic mass of neon to be 20.2. As mass spectrometers became more accurate, a third isotope of neon, neon-21, was discovered. It did not show up in Aston’s instrument because only 0.26 per cent of naturally occurring neon is 21Ne (only 26 atoms in 10 000).
As mass spectrometers have become smaller they have been used to analyse the frozen hydrocarbon surface of Titan, the largest moon of the planet Saturn, and to search for evidence of life on Mars. Mass spectrometers are used to determine the ratio of carbon-12 to carbon-14 in dead organic matter. This is the basis of carbon-14 dating.
HOW A MASS SPECTROMETER WORKS
All mass spectrometers have three basic operations:
- producing gaseous ions from a sample;
- separating the ions according to their mass (and charge):
- detecting the ions.
The type of mass spectrometer that dominated much of the 20th century was based on Aston’s apparatus and it is still in use today. A vaporised sample is injected into the instrument. The exceptional sensitivity of the instrument means that only nanograms (10-9 g) of a sample are required. This is then ionised to form positive ions by bombarding the atoms or molecules with high-energy electrons from an electron gun. The bombarding electrons knock electrons from the atoms or molecules in the sample, creating positively charged ions.
The ions are separated according to their mass and charge, first by accelerating them in an electric field of several thousand volts between two negatively charged plates. Next, they enter a strong magnetic field, which deflects them into a series of separate circular paths according to their mass/charge ratio. Positive ions with higher mass/charge ratios are deflected less than those with lower ratios. So by varying precisely the strength of the magnetic field, ions of a particular mass/charge ratio can be focused on the detector in sequential order to build up a spectrum.
On the mass spectra, the x-axis is labelled mass/charge ratio (m/e). Although 2+ ions do occur (when two electrons are removed). 1+ ions are far more abundant. For these, the charge e = +1, which makes the mass/charge ratios of the peaks equal to the relative masses of the ions. This is why you will sometimes see the r-axis labelled simply: mass.
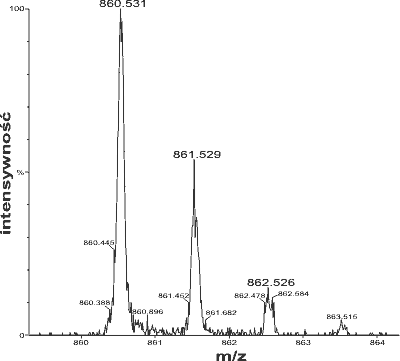
Mass spectrum of a peptide showing the isotopic distribution. Mkotl, GNU General Public License
REFERENCES
https://www.ru.nl/systemschemistry/equipment/analysis-separation/about-analysis/
http://unaab.edu.ng/funaab-ocw/attachments/474_CHM%20703.pdf
https://www.ncbi.nlm.nih.gov/books/NBK207662/
https://www.bbc.co.uk/bitesize/guides/zqqtrwx/revision/1
https://www.bbc.co.uk/bitesize/guides/zp2wrwx/revision/3
https://en.wikipedia.org/wiki/Medicinal_plants
https://www.thoughtco.com/drugs-and-medicine-made-from-plants-608413
https://thesunlightexperiment.com/blog/2018/6/7/9-famous-examples-of-drugs-that-came-from-plants
https://syrris.com/applications/drug-discovery-and-development/
https://en.wikipedia.org/wiki/Combinatorial_chemistry
https://www.pharmatutor.org/articles/combinatorial-chemistry-modern-synthesis-approach
Calculating_Molecular_Formulas_for_Compounds
Stoichiometry_and_the_Mole/5.4_Percent_Composition%2C_Empirical_and_Molecular_Formulas
https://www.chem.tamu.edu/class/fyp/stone/tutorialnotefiles/fundamentals/empirical.htm
calculating-percent-composition-and-determining-empirical-formulas
https://socratic.org/questions/how-do-you-find-molecular-formula-of-a-compound
https://chemed.chem.purdue.edu/genchem/probsolv/stoichiometry/molecular2/mf2.4.html
The Steem blockchain is currently being attacked by a central authority in order to take control of the witnesses. If you are not managing your witness votes, please consider setting @berniesanders as your witness voting proxy by clicking here to help restore the decentralization of Steem.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
@tipu curate
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Upvoted 👌 (Mana: 0/25 - need recharge?)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit