Good day, my esteemed readers. Thanks for the constant support and motivation. I do really appreciate. Still on the chemistry of cholesterol and alcohol, I will start today’s blog with the explanations on how to prepare an alcohol, then followed by the manufacture of ethanol and methanol and finally on the reactions of alcohols. So, kindly stay with me as I move on. Thanks.
PREPARATION OF ALCOHOLS
There are five ways to make alcohols, as summarized in the figure below. It follows that alcohols feature in a large number of reactions, which makes them good synthetic intermediates.
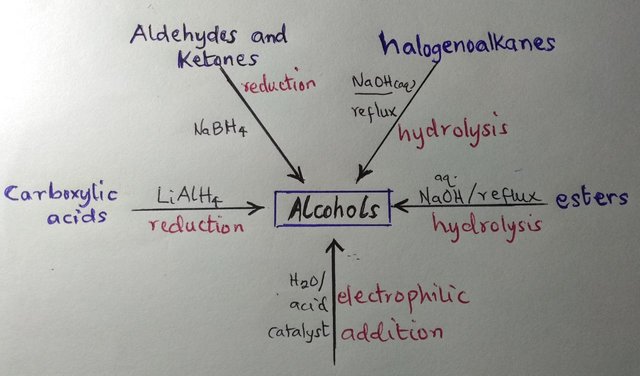
A diagram by me showing the preparative routes to alcohols
MANUFACTURE OF ETHANOL
Ethanol is a compound in great demand as a solvent, a fuel and a synthetic intermediate in the production of other chemicals. It is, of course, present in alcoholic drinks, for which it is produced by the fermentation of glucose contained in grapes, for example.
Fermentation of sugars
Ethanol is one of the oldest manufactured chemicals, going back to the time when someone is thought to have first accidentally discovered the fermentation process – possibly 5000 years ago. During fermentation (anaerobic respiration), aqueous glucose is converted into carbon dioxide and aqueous ethanol. The reaction is catalyzed by the enzymes present in yeast:
C6H12O6(aq) → 2CH3CH2OH(aq) + 2CO2(g)
The reaction is carried out between temperatures of 25 °C and 45°C; any warmer and the enzymes in yeast are denatured and no longer function as a catalyst. It is also important that oxygen is not present, because if it is, ethanol will be oxidized to produce ethanoic acid. Fermentation does not produce pure ethanol, but instead a dilute solution of ethanol is formed. To obtain higher concentrations of ethanol, the aqueous ethanol has to be fractionally distilled.
Hydration of ethene
Alkenes including ethene can be manufactured from the cracking of larger hydrocarbon molecules. Large quantities of ethene are used to manufacture ethanol in a reaction known as hydration. This is achieved by reacting ethene and steam together at 300°C and 6000 kPa (60 atmospheres) pressure in the presence of a phosphoric(V) acid catalyst. Ethene can also be hydrated in the laboratory, but different conditions must be used. Ethene is bubbled through concentrated sulphuric acid and undergoes an addition reaction to form ethyl hydrogensulphate, which is hydrolyzed to give ethanol.
Fermentation or hydration?
In the manufacture of ethanol by fermentation and by hydration, each method has its advantages and disadvantages. Ethanol made by fermentation is a renewable resource, since the sugar required for the process comes from plants that can be grown again in the following season to make more sugar. However, the hydration process uses ethene that is made by cracking of long-chain hydrocarbons obtained from crude oil, which is a non-renewable resource.
Fermentation is a batch process, so the quality of the product cannot be controlled as easily as in the continuous process of hydration. The batch process of fermentation allows a chemical company to match supply with demand fairly easily, since extra batches can be included if there is a need for extra ethanol.
Hydration of ethene produces pure ethanol, whereas fermentation produces a dilute solution of ethanol, which must be purified by distillation.
THE MANUFACTURE OF METHANOL
Methanol is used in industry and in the laboratory as a solvent and reagent; also as car antifreeze and as an engine fuel. Though an alcohol, methanol is poisonous and can cause blindness if inhaled or drunk. Until about 1925, methanol was made by heating wood in the absence of air, and collecting and condensing the volatile substances given off. This is why methanol is sometimes called wood spirit. It is now manufactured by the reaction of carbon monoxide or carbon dioxide with hydrogen. Both reactions are carried out at about 400 °C and under a pressure of some 1500 kPa. Using heterogeneous catalysts.
Alternative routes to hydrocarbon fuels
The catalyzed conversion of either carbon monoxide or carbon dioxide into methanol provides a route (at least in theory) for converting the products of combustion of a fuel into a practical liquid fuel. The methanol itself can be further converted into other useful products.
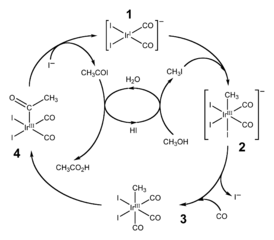
These synthetic routes provide a way for alkanes and alkenes to be synthesized without the need of a raw material such as crude oil. Perhaps more research will be needed in this direction if people are going to continue to rely on hydrocarbons as an energy source and as a raw material for the petrochemical industry.
REACTIONS OF ALCOHOLS
THE NATURE OF THE HYDROXYL FUNCTIONAL GROUP
The hydroxyl functional group in an alcohol contains one covalent bond and is attached to a carbon atom by another covalent bond. This means that the functional group can undergo two types of reaction. The first involves the breaking of the carbon-oxygen (C – O) single bond, and the second involves the breaking of the oxygen-hydrogen (O – H) single bond.
The C – O bond can be broken either by a substitution reaction or by an elimination reaction. When the O – H bond is broken, an alcohol behaves as an acid. This is because the breaking of the bond results in the transfer of a hydrogen ion. Since the oxygen atom of the hydroxyl group has two lone pairs of electrons, an alcohol can also behave as a nucleophile, reacting with a molecule that has an electron-deficient centre.
Finally, an alcohol can take part in redox reactions. The large range of reactions available to this functional group helps to explain why alcohols are very good synthetic intermediates.
SUBSTITUTION REACTIONS: HALOGENATION OF ALCOHOLS
The conversion of an alcohol into a halogenoalkane is a substitution reaction that involves the swapping of a halogen atom for a hydroxyl group. However, a halide ion is not a good enough nucleophile to substitute a hydroxyl group directly. The strength of the C – O bond is a great barrier to the substitution reaction.
The only way that the substitution reaction can succeed is if the C – O bond is substantially weakened. An acidic catalyst can be used to do this. A lone pair of electrons on the oxygen atom is donated to a proton in an acid-base type interaction. The intermediate formed has a very weak C – O bond, since the oxygen has developed a positive charge. Nucleophilie substitution by a bromide ion is now very easy, with the water molecule as the leaving group. Normally, the reaction is carried out by refluxing togefher a mixture of alcohol, sodium bromide and concentrated sulphuric acid. The concentrated sulphuric acid and the sodium bromide together generate concentrated hydrobromic acid:
For the substitution of the hydroxyl group by a halogen atom, the corresponding acid can be used. Iodo compounds are prepared by refluxing the alcohol with concentrated hydroiodic acid, bromo compounds by refluxing with concentrated hydrobromic acid and a trace of concentrated sulphuric acid as a catalyst, and chloro compounds by refluxing with concentrated hydrochloric acid using zinc chloride as a catalyst.
There are alternative ways to achieve this substitution. All of them involve weakening the C – O bond by a reaction with a phosphorus atom. Typically phosphorus(V) chloride, phosphorus(III) bromide, or a mixture of phosphorus and iodine (essentially phosphorus(III) iodide) is refluxed with the alcohol to obtain the corresponding halogenoalkane. Representing an alcohol by the formula ROH, where R is an alkyl group, the equations for these three reactions are:
PCl5 + ROH → RCl + POCl3 + HCl
PBr3 + 3ROH → 3RBr + H3PO3
PI3 + 3ROH → 3RI + H3PO3
Phosphorus(V) chloride for working out structures
Phosphorus(V) chloride reacts with hydroxyl groups in alcohol and carboxylic acids to produce fumes of hydrogen chloride, which are easily detected. So this reaction is used to analyze compounds to identify hydroxyl groups and establish whether they are alcohols or carboxylic acids.
ELIMINATION REACTIONS: DEHYDRATION TO FORM ALKENES
As I have already described, alcohols can react by substitution and elimination. The elimination reaction is more frequently referred to as dehydration, since it involves the loss of a molecule of water as well as the formation of an alkene. In dehydration. An alcohol is heated with a suitable catalyst such as concentrated sulphuric acid or concentrated phosphoric acid. Alternatively, the hot alcohol vapour is passed over a heated aluminium oxide catalyst.
Laboratory dehydration
In the laboratory, it is convenient to take an alcohol and reflux it with a dehydrating agent. Concentrated suphuric acid can be used, but concentrated phosphoric(V) acid is usually preferred, because it minimizes the number of side products formed. With some alcohols, several alkenes may be produced, but normally the most-substituted alkene is the major product.
ALCOHOLS AS NUCLEOPHILES
The oxygen atom of the OH group of an alcohol can donate a pair of electrons to make a covalent bond, which allows the group to act as a nucleophile.
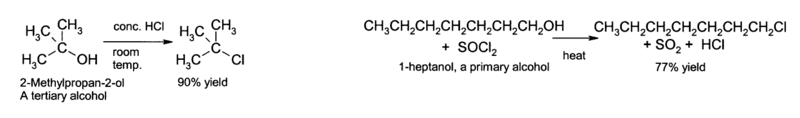
Some simple conversions of alcohols to alkyl chlorides. Maksim, Public Domain
Alcohols are poor nucleophiles, but they can react with electron-deficient centres provided the deficiency is sufficiently high. Typically, alcohols behave as nucleophiles in their reactions with carboxylic acids or their derivatives. Probably the most important reaction is that with a carboxylic acid to form an ester. Esters are sweet-smelling substances used in perfumes and in food flavourings.
REFERENCES
https://en.wikipedia.org/wiki/Alcohol
https://en.wikipedia.org/wiki/Ethanol_fermentation
https://www.thoughtco.com/how-ethanol-is-made-1203781
https://www.chemguide.co.uk/physical/equilibria/ethanol.html
http://www.docbrown.info/page04/OilProducts09.htm
https://www.bbc.co.uk/bitesize/guides/zyf82hv/revision/3
https://www.chemguide.co.uk/organicprops/alcohols/manufacture.html
https://en.wikipedia.org/wiki/Methanol
https://www.sciencedirect.com/topics/engineering/production-of-methanol
http://www.essentialchemicalindustry.org/chemicals/methanol.html
https://en.wikipedia.org/wiki/Hydroxy_group
https://en.wikipedia.org/wiki/Alcohol
Chem. Libretexts: Properties of Alcohol
https://www.britannica.com/science/alcohol/Reactions-of-alcohols
http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch04/ch4-5.html
https://www.chemguide.co.uk/mechanisms/elim/dhethanol.html
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch05/ch5-2.html

This reminds me of my secondary school chemistry. You are doing a great job simplifying these reactions.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks for coming by. @gentleshaid
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit