A colorimeter is a simple form of visible spectrometer. A fixed band of wavelengths of visible light is selected using a filter. This is then passed through the solution under test. The light that has not been absorbed by the solution is transmitted to a photocell. The light generates an electric current, which is measured by a meter. The more light that is transmitted by the solution, the greater the electric current produced. However, most colorimeter meters are calibrated so that they record the light absorbed by the solution rather than the light transmitted. The absorbance of a solution is proportional to the concentration of the coloured compound in the solution.
The colorimeter is set to zero by inserting a tube of water and pressing a zeroing button. Standard solutions of known concentrations of a species whose concentration you wish to determine are then placed in the colorimeter and their absorbances measured. A graph of absorbance against concentration is then plotted. This is called a calibration curve. The absorbance of a solution of unknown concentration can now be measured and the reading checked against the calibration curve to determine the concentration of the particular species under investigation. The addition of aqueous potassium thiocyanate to a solution of aqueous iron(III) ions [Fe(H2O)63+ causes a distinctive blood-red complex to form. We can use the colorimeter to determine the formula of this complex.
The first task is to select a filter for the colorimeter that will allow the solution to absorb most strongly. Since the iron thiocyanate complex is red it will absorb most in the blue wavelengths of the spectrum, so a blue filter is chosen. Solutions to be analysed are next made up to give different molar proportions of Fe3+(aq) and SCN- (aq): 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, etc. If formula of the complex is [Fe(H2O)5(SCN)2+, then 1 mole of [Fe(H2O)6]3+ would need to be added to 1 mole of SCN- ions, that is the 5:5 mixture. This is, in fact, the correct formula. If these different molar proportions were placed in turn into the colorímeter, a graph similar in shape. In such a graph, you will notice that the maximum absorbance corresponds to the molar proportions:
5Fe3+ (aq):5SCN- (aq)
which is the 1 mole:1 mole ratio.
THE COLOURS OF GEMSTONES
Some of the most beautiful and highly prized examples of the colours that transition metal ions can impart are found in gemstones. In many cases, the transition metal ions are actually impurities. For example, the deep red of rubies comes from traces of Cr3+ ions embedded in a lattice of aluminium oxide. Cr3+ ions have the same charge as Al3+ ions and are about the same size, and they occupy about 5 per cent of the Al3+ posítions. Emeralds are coloured green also because Cr3+ ions are present. This time the Cr3+ ions are embedded in a lattice that contains large silicate anions Si6O1812- together with the cations Be2+ and Al3+.
So how can Cr3+ impart different colours? The answer lies in the splitting of the d orbitals. There are three 3d electrons in Cr3+. One of these d electrons becomes excited by absorbing a photon of light with a wavelength in the visible spectrum. The wavelengths that are transmitted through the gemstone are predominantly red, which is why a ruby is red. In the case of emerald, the different environment of the Cr3+ ion leads to a different energy gap between the two sets of d orbitals, one that allows the transmision of blue-green light while absorbing violet, yellow and red wavelengths.
Other precious gemstones are coloured by different transition metal ions. The blue of sapphire results from Ti4+, V3+ or Co3+ ions. Aquamarine, named after the colour of a tropical sea, owes its pale blue colour to Fe3+ ions. The Fe3+ ion also gives a yellow colour to topaz and a red colour to garnet. The purple of amethyst is caused by Mn3+ ions.

THE COLOURS OF GEMSTONES. Brillanten, CC BY-SA 3.0
THE AMAZING MONASTRAL BLUE
Through your studies in science you may have realised that many important discoveries occur by accident. An example in this post is the discovery of the first synthetic dye by William Perkin, which he called mauve (see here). The English writer Horace Walpole invented a term for this in 1754; he called accidental discoveries that lead to happy outcomes serendipity. The word has its origin in a fairy tale about the three princes of Serendip who were constantly making chance but fortunate discoveries. The story of the blue pigment Monastral Blue is an example of serendipity.
The story begins in 1928 when a researcher at Scottish Dyes Ltd, A. G. Dandridge, noticed blue crystals on the lid of a glass-lined iron vat designed to make the compound phthalimide from molten phthalic anhydride and ammonia. Phthalimide is a white compound and was a necessary compound in the manufacture of some dyestuffs. Sometimes, when the glass lining became damaged, similar blue crystals would form on the sides of the vat and contaminate the white molten phthalimide. It is fortunate that Dandridge was curious enough to examine and analyse these crystals. He realised that they were made of a compound of iron and he named it iron phthalocyanine. He also understood that it had potential as a pigment.
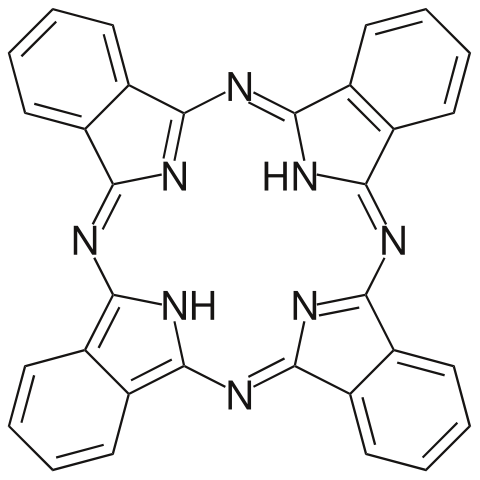
Phthalocyanine. Choij, Public Domain
The ring structure that surrounds the iron atom is very similar to those found in nature, called porphyrins. Iron porphyrin occurs in haemoglobin and the plant pigment chlorophyll also contains a porphyrin ring. The ring structures contribute to the colour of the molecules. If you examine the iron phthalocyanine’s structure closely, you will see it has a conjugated structure that forms a large delocalised system. Once the structure of iron phthalocyanine was known, researchers at Imperial College, London, set about making other phthalocyanines, the best of which was copper phthalocyanine, first made in 1934. This intense blue pigment goes under the trade name Monastral Blue. It is used extensively by printers, paint manufacturers and to colour plastics. It is non-toxic and is even used to stain cells in the sclera membrane of the eye to facilitate laser surgery. The reason it is so useful in laser treatments is that Monastral Blue strongly absorbs in the infrared region of the spectrum.
SUMMARY
After studying this chapter, and the two previous ones on the chemistry of colour, you should know that:
- Electron energy levels exist in atoms and molecules. Electron transitions between these levels emit or absorb radiation.
- Colour arises when a substance absorbs or emits electromagnetic radiation partly in the visible spectrum.
- Absorption spectra record the wavelengths of photons that excite electrons and so cause them to jump to higher energy levels. Emission spectra record the wavelengths of photons emitted when excited electrons return to lower energy levels.
- Atomic emission spectroscopy can be used to determine the concentrations of metal ions in biological fluids, such as blood serum.
- Dyes are soluble in the medium in which they are applied; pigments are insoluble.
- Azo dyes are produced by coupling reactions, such as that between benzenediazonium chloride and phenol.
- The functional group of an azo compound is the azo group, -N=N-.
- Electron transitions in organic molecules give rise to ultraviolet or visible absorptions, because the molecules contain double or triple bonds, delocalised systems or lone pairs of electrons.
- Ultraviolet-visible spectroscopy is possible because when the outer electrons of atoms or ions in compounds are excited they absorb in the ultraviolet or visible part of the spectrum.
- Conjugated systems have alternating double and single bonds; the delocalisation that occurs lowers the energy of electron transitions, so conjugated compounds may appear coloured.
- The part of a molecule responsible for absorbing coloured radiation is called the chromophore. It is usually an extended delocalised electron system.
- The colour changes of acid-base indicators results from a change in the delocalisation of the chromophore when H+ ions are added or removed.
- The colour of a transition element’s ions results from a difference in energies between d orbitals caused by the ligands around the metal cation. This splitting allows d-d electron transitions.
- Factors that affect d-d splitting include the oxidation state of the metal, the nature and number of the ligands, and the arrangement of the ligands around the metal cation.
- Complexes of Zn2+ and Cu+ are white because the 3d subshell is full.
- The formula of a complex can be determined using colorimetry.
REFERENCES
https://www.sciencedirect.com/topics/engineering/colorimeter
https://www.azom.com/article.aspx?ArticleID=13983
https://en.wikipedia.org/wiki/Colorimeter_(chemistry)
https://www.azosensors.com/article.aspx?ArticleID=324
https://www.rocksandco.com/gemstone-information/gemstone-colours/
https://www.palloys.com.au/technical/gemstone-colours/
https://www.ch.imperial.ac.uk/rzepa/blog/?p=3641
https://en.wikipedia.org/wiki/Phthalocyanine_Blue_BN
http://shipseducation.net/modules/scimath/polymer1.htm
https://pffc-online.com/material-science/1406-paper-great-discovery-mess
https://www.sigmaaldrich.com/catalog/product/aldrich/379549?lang=en®ion=US
https://pubchem.ncbi.nlm.nih.gov/compound/Iron-phthalocyanine
https://www.nature.com/articles/136862c0
https://en.wikipedia.org/wiki/Phthalocyanine
https://www.britannica.com/science/phthalocyanine
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I love reading about serendipity. Those are always cool stories :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks, @lemouth.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
I shared your outstanding contribution on color chemistry, from my Twitter: account: https://twitter.com/lupafilotaxia/status/1216407453891514368
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks, @lupafilotaxia.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit