INTRODUCTION: Envirocats
Benzene is one of the most industrially important organic molecules. It features in the manufacture of petrochemicals, which are solvents, detergents, insecticides, dyes and polymers. The benzene molecule is normally unreactive, so to make these compounds requires special conditions such as high pressures and high temperatures. Almost all the reactions of benzene require catalysts, many of them hazardous environmental pollutants that can pollute water supplies and poison the organisms in them.
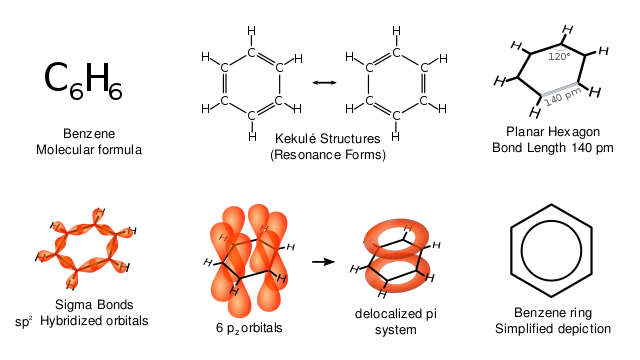
The various representations of benzene. Vladsinger, CC BY-SA 3.0
So there is an urgent need to develop environmentally friendly catalysts envirocats – that can be separated easily by filtration from mixtures after they have reacted. Envirocats are based upon clay materials, which are made acidic by adding metal salts. Since they can be filtered from any aqueous effluent, envirocats can be prevented from harming water supplies and aquatic life. In addition, since envirocats are solid, their controlled disposal is easy. They have the economic benefit of conventional catalysts, too – envirocats can be used again and again, but eventually they lose their catalytic activity.
AROMATIC COMPOUNDS AND THEIR USES
In 1825, a young scientist at the Royal Institution of London was Asked to remove the oily residue that collected in the gas cylinders of gas lamps and analyze it. He found that the residue contained a previously unknown hydrocarbon, for which the molecular formula was later shown to be C6H6. The scientist was Michael Faraday, one of the greatest scientists of the 19th century, though his name is seldom linked with organic chemistry.
The molecule of every aromatic compound contains at least one benzene ring. The name ‘aromatic’, meaning ‘with an odour or smell’, was given to these compounds because the first few to be discovered and used do, indeed, have odours. However, now we know that this was just a coincidence. Many aromatic compounds have no odour, whereas others have a particularly nasty smell. Also, some fragrant compounds do not contain a benzene ring. Aromatic compounds can contain other functional groups as well as alkyl groups.
The benzene ring is based upon a hexagonal arrangement of six carbon atoms and six delocalized π electrons, which give the ring unique stability. The hydrogen atoms in the benzene molecule can be replaced by alkyl, aryl or other functional groups. Note the way in which the benzene ring is represented in compounds: it is a skeletal formula. The atomic symbols are not included, so remember that the benzene ring consists of six hydrogen atoms and six carbon atoms. The significance of the circle drawn inside the hexagon will be explained later on.
SOME USES OF AROMATIC COMPOUNDS
The figure below features a compound that have one thing in common: the benzene ring, They are therefore all called aromatic compounds. All but two (2-phenylethan-1-ol and naphthalene) have to be synthesized industrially. The starting point for some of these compounds is benzene, C6H6, so the benzene part of their carbon skeleton is in place. Chemical changes are then needed to substitute one or more of the hydrogen atoms with one or more of the alkyl or aryl groups.
Toluene. Luigi Chiesa, Public Domain.
STRUCTURE OF BENZENE
Benzene is one of the class of hydrocarbons known as arenes. Arenes are unsaturated hydrocarbons, characterized by great stability and non-reactivity, which distinguishes them from alkenes. All arenes contain a delocalized π system of electrons in their benzene rings. Arenes are strictly hydrocarbons. Aromatic compounds can contain other functional groups.
KEKULÉ AND THE FIRST STRUCTURE OF BENZENE
Following the discovery of benzene, chemists had the challenge to try and determine its structure. In the early 19th century, benzene’s molecular formula, C6H6 did not fit the way that chemists thought carbon atoms bonded in organic molecules.
It was 40 years later that August Kekulé proposed the ring structure of benzene, the first time a chemist realized that carbon atoms could form rings as well as chains. His brilliantly original idea was attributed to a dream he had about a snake biting its tail. Kekulé drew the structure of benzene as cyclohexa-1,3,5-triene.
Kekule’s structure suggests that benzene has three single bonds and three double bonds, so that it should react in the same way as cyclohexene and be able to undergo electrophilic addition. It further suggests that benzene should have long carbon-carbon bonds, C-C, and short carbon-carbon bonds, C=C.
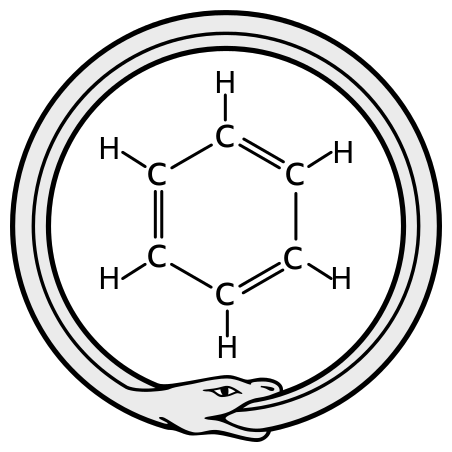
The ouroboros, Kekulė's inspiration for the structure of benzene. Haltopub, CC BY-SA 3.0
Faults in Kekulé’s model
Structural investigations using X-ray crystallography and infrared spectroscopy show that the benzene molecule is a regular hexagon of carbon atoms, with the lengths of all six carbon-carbon bonds equal to 140 pm. This value lies between the average bond length for a single bond, 154 pm, and the average bond length for a double bond, 134 pm. [Note: The abbreviation pm stands for picometre, a unit of length equal to 10-12 m].
A further complication is that benzene does not behave like an alkene. It reacts by electrophilic substitution rather than by electrophilic addition (would be discussed later in the article). There are couple of differences between cyclohexene, an alkene with a six-carbon ring, and benzene. The reactions of the two compounds too are very different. This suggests that benzene cannot be an alkene and therefore cannot have Kekulé’s structure of cyclohexa-1,3,5-triene.
Enthalpy change of hydrogenation
Unsaturated compounds, such as alkenes, react with hydrogen under pressure in the presence of a nickel catalyst to form alkanes. This reaction is known as hydrogenation.
The enthalpy change of hydrogenation is defined as the enthalpy change that occurs when one mole of an alkene reacts with hydrogen to form an alkane. Studies of the hydrogenation of different alkenes show that for a molecule with one C=C bond, the enthalpy change of hydrogenation is about -120 kJ mol-1. For a molecule with two double bonds, the enthalpy change of hydrogenation is about -240 kJ mol-1.
Kekule’s structure for benzene has three C=C bonds, so we might expect the enthalpy change of hydrogenation for benzene to be about -360 kJ mol-1. However, experimental data shows that its value is only -208 kJ mol-1, which is a considerably smaller enthalpy change than the theoretical value. The only conclusions that can be drawn from the experimental data are that the benzene ring does not contain three C=C bonds, and that benzene is more stable than cyclohexa-1,3,5-triene.
MODERN THEORIES OF THE BENZENE MOLECULE
The value of the enthalpy change of hydrogenation of benzene is conclusive evidence that the benzene ring does not have the C=C bonds that Kekulé predicted. In addition, the enthalpy change transfers much less energy to the surroundings than his model predicts, which indicates that the bonding of the carbon atoms in the benzene ring is much stronger than Kekulé’s model predicted, and so benzene is more stable.
Resonance structure model of the benzene molecule
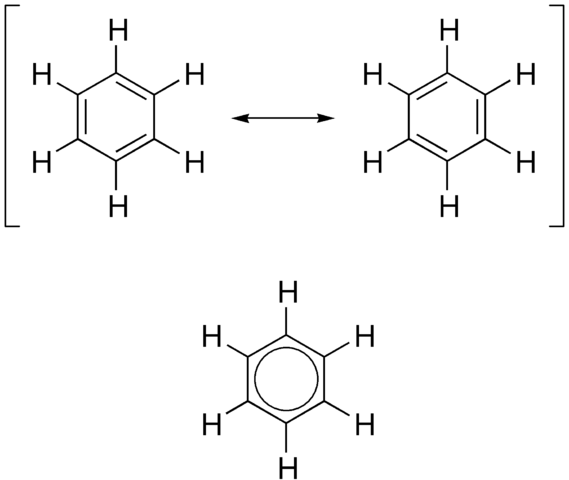
Kekulé’s model of benzene is inaccurate in the ways we have seen, and does not match the known physical dimensions of the benzene molecule. The figure below shows the structure of a benzene molecule as a hybrid of two Kekulé structures. The structure of benzene is called a resonance hybrid. Despite the name ‘resonance hybrid, this model does not imply that the hybrid structure is continually changing from one to the other, but rather that it is a cross between the two structures, and possesses some of the characteristics of each. This means that each carbon-carbon bond has some single-bond and some double-bond character. For this model it is often better to draw the structure of the benzene ring as shown in the figure.
The circle represents a bond that contains six π electrons that are delocalized around the ring. This model has now been superseded by the orbital overlap model.
Orbital overlap model
Covalent bonds are formed when atomic orbitals overlap. The C-H bond is a α bond formed by the overlap of a hydrogen 1s atomic orbital with an atomic orbital on carbon. The C-C bond is a α bond formed by the overlap of two atomic orbitals, one from each carbon atom.
In benzene, there is a third type of bond, which is formed by the side-to-side overlap of six 2p atomic orbitals on carbon. This forms an orbital that spreads, rather like a ring doughnut, above and below the plane of the carbon atoms that possess the six electrons. These electrons are not found between any particular atoms and so are known as delocalized π electrons.
Stabilisation energy
Earlier in the chapter, we saw that the enthalpy change of hydrogenation of benzene is about 150 kJ mol-1 less than expected from the Kekulé model. This suggests that benzene is about 150 kJ mol-1 more stable than cyclohexa-1,3,5-triene (Kekulé’s structure). This energy difference is sometimes referred to as the stabilisation energy of benzene.
The idea of stabilisation energy means that to break the benzene ring you need more energy than is predicted from Kekulé’s model.
Structural formula for benzene
The figure above also shows three ways to represent the displayed formula for benzene. Note that neither the hydrogen atoms nor the carbon atoms are shown. It is a skeletal formula. Every time you see one of these skeletal formulae of the benzene ring, remember that there are six hydrogen atoms attached to the ring. The circle inside the third hexagon represents the delocalized electrons. Each representation in has its advantages and disadvantages. The first two show three discrete double bonds (which do not exist in benzene), and the bottom representation shows that some electrons are delocalized, but not how many. Nowadays, the third one is the most accepted way to represent the structure of benzene.
NAMING AROMATIC COMPOUNDS
Arenes is the group name we give to benzene and the hydrocarbons that are just benzene rings joined together (fused). Arenes have similar chemical properties because their benzene rings have delocalized electrons. We have already described aromatic compounds in general as those that contain at least one benzene ring. Aromatic compounds are not necessarily hydrocarbons, but can contain a wide variety of other functional groups.
SUBSTITUTED ARENES
To name aromatic compounds, we use the name of the parent arene together with a prefix or suffix to indicate the groups of atoms attached to the benzene ring. So, for example, the prefix ‘methyl’ in the name methylbenzene indicates that the methyl group has replaced one of the hydrogen atoms in the benzene ring. When more than one hydrogen atom is replaced by methyl groups, as in dimethylbenzene, we specify the positions of the methyl groups by numbering the carbon atoms. So there are three structural isomers that could be called dimethylbenzene: 1,2-dimethylbenzene, 1,3-dimethylbenzene and 1,4-dimethylbenzene.
1.3-dichlorobenzene has a benzene ring with two chlorine atoms in place of hydrogen atoms. The first chlorine is attached to the carbon we number as carbon 1, and the second to carbon 3. A benzene ring is numbered clockwise or anticlockwise, depending on which direction gives the lower position numbers. 1-chloro-2-methylbenzene has two different groups attached to the benzene ring. Notice that we number the carbon attached to the chloro group as carbon 1, rather than the carbon attached to the alkyl group. (Halogens are named before alkyl groups.)
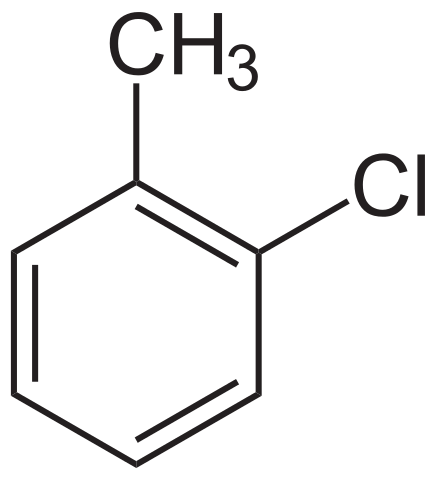
1-chloro-2-methylbenzene. NEUROtiker, Public Domain.
Phenyl group
The phenyl group is C6H5. It is found attached to carbon chains and to other functional groups. The phenyl group is an example of an aryl group. An aryl group is the aromatic equivalent of an alkyl group and always contains at least one benzene ring. In phenylamine, for example, the phenyl group is attached to the amine functional group. Phenylamine has the formula C6H5NH2.
Compounds with some substituents (groups) on the benzene ring retain their traditional names. We refer to phenol instead of hydroxybenzene. In phenol, the carbon attached to the OH group is called carbon 1.
Benzoic acid and benzaldehyde
Benzoic acid and benzaldehyde are two more aromatic compounds that have retained their older, pre-systematic names. Note carefully that both of these compounds contain seven carbon atoms rather than six, because the functional groups contain a carbon atom.
Thanks for coming.
REFERENCES
https://en.wikipedia.org/wiki/Aromaticity
https://courses.lumenlearning.com/introchem/chapter/properties-of-aromatic-compounds/
https://en.wikipedia.org/wiki/Aromatic_hydrocarbon
https://www.britannica.com/science/Kekule-structure
https://en.wikipedia.org/wiki/Chlorotoluene
https://en.wikipedia.org/wiki/Toluene
https://www.chemguide.co.uk/basicorg/bonding/benzene1.html
https://www.thestudentroom.co.uk/showthread.php?t=4599510
http://202.127.145.151/siocl/cdbank/WebHelp/Beilstein/brefhtml/hhdg.htm
https://emilycblog.wordpress.com/2015/06/09/problems-with-kekules-proposed-structure-of-benzene/
https://en.wikipedia.org/wiki/Arene_substitution_pattern
Congratulations! I just stopped by to say that your post has been selected as a daily Featured Post of my personal curation project! You can find the daily Featured Post HERE.
I upvoted your contribution and I put it on the list because to my mind your post is what I call a quality content!
I am @miti, a manual curator that shall make available all his Steem Power to authors deserving of support. Let's make STEEM great again!
Have a nice day and keep up the good work!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thanks, @miti.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
You're welcome!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations @empressteemah! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit