It's hard to imagine how small a atom is. For example, the thickness of ordinary paper is about half a million atoms. And one atom is just as small compared to an apple, as little apple as compared to the Earth. And you probably will be surprised that chemists can actually see atoms. Certainly not with eyes but with help of very precise tools. But how? To answer this question, let's turn to history.
The existence of atoms directly stretches from ancient Greece - at that time the philosopher Democritus stated that all matter on our Earth is made of the smallest indivisible particles that he called atoms, which is translated as indivisible. And the philosopher Plato at the same time even mistakenly decided that the difference of substances is a different form of atoms like pyramids or cubes. However, today from elementary school lessons we know that an atom - is the smallest particle of a chemical element that is invisible to the eye and is capable of independent existence and possesses its properties. But then it was not possible to prove it.
The first evidence of the existence of atoms appeared around the beginning of the 1800s, when the British chemist John Dalton discovered that substances contain whole numbers of atoms. Dalton, in spite of all, suggested that there can not be half an atom or one and a half atoms, but only a whole atom. That's why now the water formula looks like H2O, not H√3.17O. And if you think about it, it's really very difficult nowadays to imagine modern chemistry without Dalton's insight.
But nevertheless and in his days this assumption was controversial. After all, at that time chemists could not see the atoms, so many perceived them as negative numbers or ideal gases that do not exist in reality and are only needed in calculations. And this is not a joke, for example, even Dmitry Ivanovich Mendeleev - father of the periodic system of chemical elements for many years refused to believe in the existence of atoms. Or maybe it was worth looking for atoms under a microscope?
The essence of Mendeleev's discovery is that with the growth of the atomic mass, the chemical properties of elements do not change monotonically, but periodically. After a certain number of elements of different properties, the properties begin to repeat. So, potassium is similar to sodium, fluorine to chlorine, and gold is similar to silver and copper. The periodic table was finally established only when Mendeleyev's words were confirmed - the scientists discovered several new chemical elements that fully corresponded to the previously described properties.
This is a very good question, but in fact you can see something in the microscope only if the wavelength of the light wave in the microscope is not greater than what we are looking at. And unfortunately the length of visible light is thousands of times larger than the atom itself. Therefore, chemists had to wait for the appearance of rays with a shorter wavelength, for example, such as X-rays.
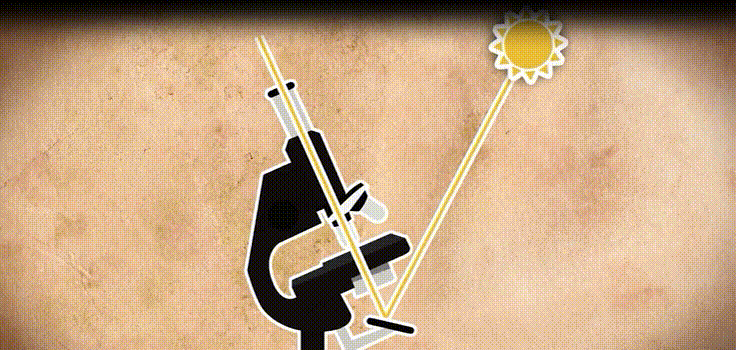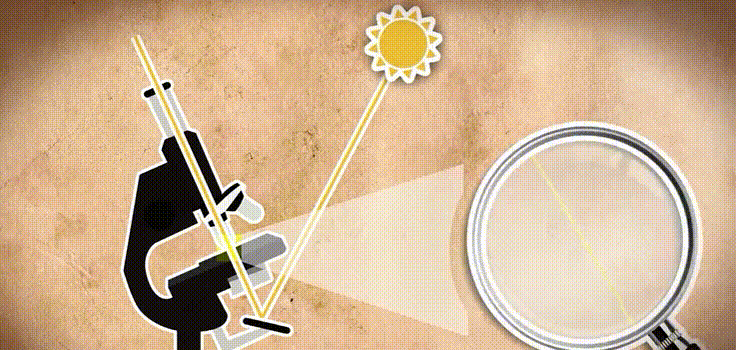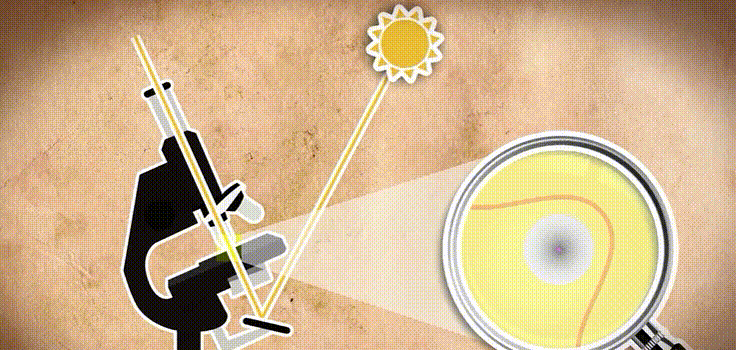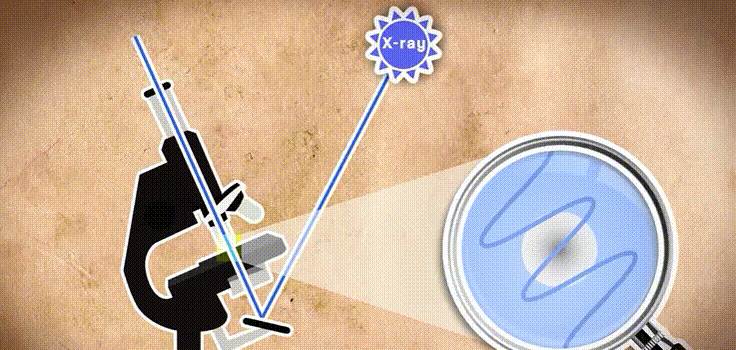
And finally, in 1890, the German scientist Wilhelm Roentgen discovered X-rays. Engaged in spectrography and studying the sun's rays (I told about this here), he managed to find out that the photographs made with the help of the rays opened by him allow us to see through the objects. At some point, Rengen thought he was crazy, and he can be understood. But today we all know about these rays from our campaigns to dentists or doctors. However, chemists use X-rays not to see through the objects.
Instead, they bombard these rays with things such as crystals, because they are a dense structure with layers of atoms. The rays strike the atom of the crystal and are reflected back. And some reflect further and pass from the atoms of the second layer or the third and so on. Once these rays have been reflected, they return to the detector screen as if as a bouncing ball when playing ping pong. Based on the collisions in the resulting figure, scientists can process everything in the opposite direction and build a 3D arrangement of atoms in the crystal. A reflection and interaction of light rays is called - diffraction.
X-ray diffraction, sometimes called x-ray crystallography, has helped to get dozens of Nobel Prizes in the early chemistry since the 1920s. This also led to one of the greatest discoveries in the history of science - the discovery of the structure of DNA.
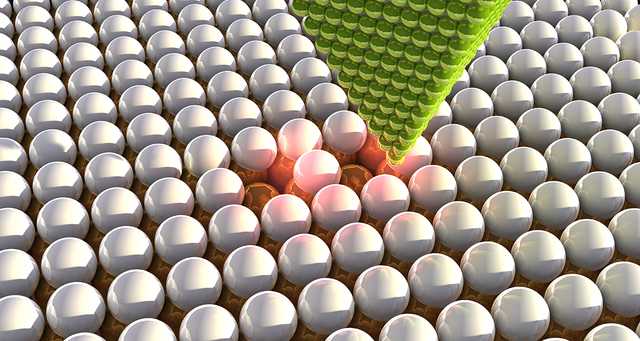
X-rays allowed the chemists to look at the structure of atoms, and the scanning tunneling microscope (STM) finally showed the atoms themselves. Instead of reflecting light from something, STM leads a sharp needle above the surface - it's like a braille chemical font, but only the tip of the needle does not touch anything. And while the needle moves the scientists reconstruct the landscape of atoms. So in the early 1980s, at last, it was possible to see individual atoms. And they were not at all like cubes or pyramids, but were spheres of various sizes.
However, in 1989, the chemist Ahmed Zewail managed to go a long way in studying immobile atoms - he invented a tool that allows them to observe them in motion. He invented the fastest camera shooting laser pulses at a speed of several femtoseconds - a few quadrillionths of a second. While Zewail's laser flew like a stroboscope, the camera took pictures, and then these pictures were viewed in slow motion.
For his achievements in the field of chemistry, Zewail received the Nobel Prize in 1999, and the field of his studies was called - femtochemistry. Since then femtochemistry has provided an understanding of everything - from the depletion of the ozone layer to the operation of the retina. So it turns out that the ancient Greeks mistakenly invented bizarre forms of atoms, but it took 2,400 years before scientists could see the atoms and study their behavior.
UpVoted well-informed article keep it up
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thank you @tokoya, I'm very glad that you liked the material. I really want chemistry, as well as other sciences in schools to teach like this :)
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
The post is really educating.....Thanks
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Thank you.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by floxxy from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit
Downvoting a post can decrease pending rewards and make it less visible. Common reasons:
Submit